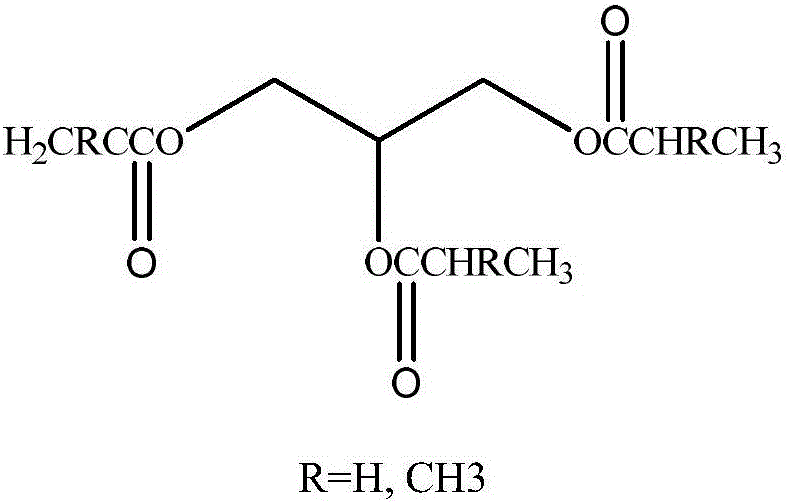
Method for synthesizing (methyl) acrylate glyceride through catalysis of calcium glyceroxide
A technology of glycerol acrylate and alkyl acrylate, which is applied in chemical instruments and methods, catalytic reactions, catalysts for physical/chemical processes, etc., can solve the problem of low activity selectivity of basic catalysts, difficult separation and purification of products, and poisoning and deactivation of dosage. and other problems, to achieve the effect of stable and easy separation of products, short process flow, and not easy to polymerize
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 glyceryl acrylate
[0032] In the there-necked flask equipped with electromagnetic stirring, thermometer, rectifying column, receiving tube, and receiving bottle, add 77.5g (0.9mol) methyl acrylate, 9.2g (0.1mol) of glycerol and 0.5g calcium glycerol, heat and reflux and stir, Make the methanol produced by the reaction continue to be azeotropically distilled from the upper end of the rectifying column, and react until no methanol is distilled off, then filter the reaction solution, reclaim the catalyst, distill off the raw material methyl acrylate, and obtain 28.3 g of the product, in which glyceryl diacrylate and The glycerol triacrylate content was 22.5% and 42.3%, respectively.
Embodiment 2
[0033] The preparation of embodiment 2 glyceryl acrylate
[0034] Add a magnetic stirrer bar in a 250mL four-necked flask equipped with an air inlet, a copper mesh packing rectification column and a temperature measuring device, add 9.2g (0.1mol) glycerol, 0.50g (0.5% glycerol mass) and 0.05g glycerol calcium For p-hydroxyanisole, raise the temperature to the reaction liquid at 60°C, put 77.5g of methyl acrylate into the constant pressure dropping funnel and add it dropwise within 2-3 hours, let the air in and raise the temperature until the reaction liquid refluxes, and take samples regularly during the reaction The end point of the reaction was analyzed by gas chromatography. After the reaction reaches the end point, add 0.1g of neutral activated carbon and reflux at about 50°C for 0.5h-1h. After the end, the reaction solution is filtered under reduced pressure, and the filtrate is rotary evaporated to obtain 31.4g of the product. The contents of glyceryl diacrylate and glyc...
Embodiment 3
[0035] The preparation of embodiment 3 (meth) glyceryl acrylate
[0036] In a three-necked flask equipped with electromagnetic stirring, a thermometer, a rectifying column, a receiving tube, and a receiving bottle, add 90.1 g (0.9 mol) of methyl acrylate, 9.2 g (0.1 mol) of glycerol and 0.5 g of calcium glycerol, and heat to reflux and stir. Make the methanol produced by the reaction continue to be azeotropically distilled from the rectifying column upper end, react until no methanol is distilled off, filter the reaction solution, reclaim the catalyst, distill the raw material methyl acrylate to obtain 29.6 g of product glyceryl acrylate, di(methyl acrylate) Glyceryl) acrylate and glyceryl tri(meth)acrylate content were 37.5% and 40.4%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com