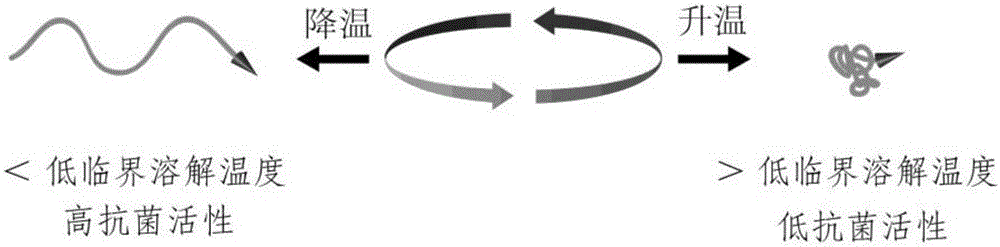
Antibacterial agent activity calling switch based on poly(N-isopropylacrylamide)thermotropic conformation reversible transition
A technology of isopropylacrylamide and antibacterial agent, which is applied in the field of antibacterial agent activity call switch, can solve the problems of separation and purification difficulties, few expression products, high cost, etc., and achieve the effects of broad antibacterial spectrum, shielding and strong antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
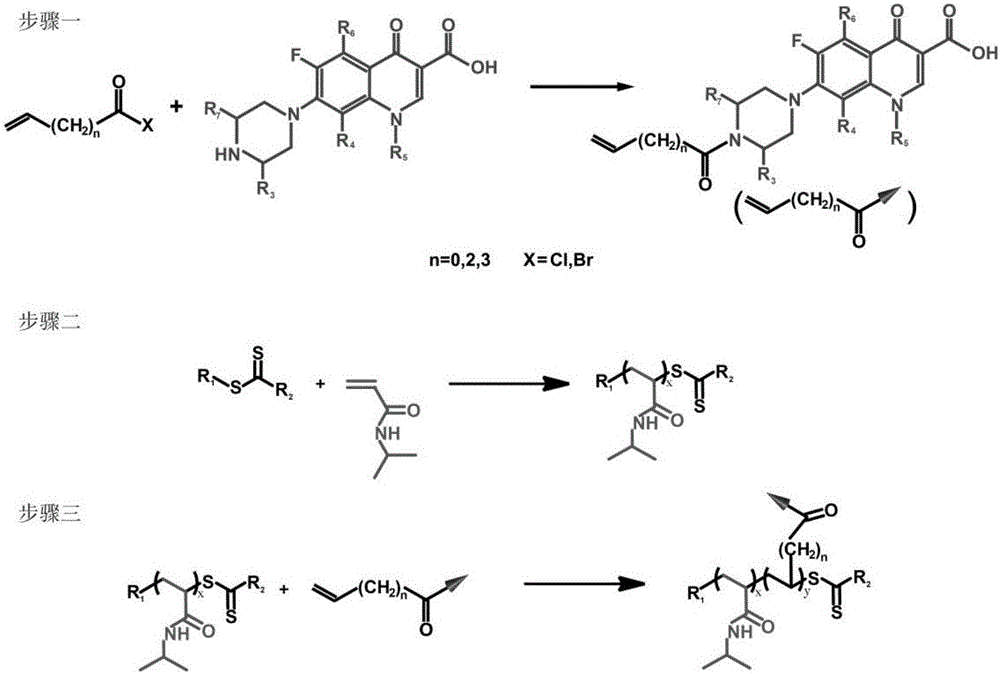
[0025] (1) Vinylation of ciprofloxacin: Mix 10 parts of ciprofloxacin, 4 parts of triethylamine and 300 parts of dichloromethane evenly, stir at 0°C for 30 minutes, then add dropwise under continuous stirring and nitrogen protection 4 parts of acryloyl chloride, after the dropwise addition, the temperature was raised to 20°C for 1 hour reaction; after the reaction, the above mixture was poured into n-hexane, and the precipitate was repeatedly washed with water and dried to obtain vinylated ciprofloxacin;
[0026] (2) N -Reversible addition-fragmentation chain transfer polymerization of isopropylacrylamide: 1 part of 2-phenyl-2-propylbenzodisulfide, N - Mix 90 parts of isopropylacrylamide, 0.07 parts of azobisisobutyronitrile and 120 parts of tetrahydrofuran evenly, seal, cycle freezing-thawing to remove oxygen, then raise the temperature to 55°C for 4 hours under continuous stirring and nitrogen protection, After the reaction was completed, the above mixture was poured into ...
Embodiment 2
[0032] (1) Gatifloxacin vinylation: Mix 15 parts of gatifloxacin, 6 parts of pyridine and 400 parts of tetrahydrofuran evenly, stir at 2°C for 45 minutes, then add 4-pentene dropwise under continuous stirring and nitrogen protection 6 parts of acid chloride, after the dropwise addition, the temperature was raised to 30°C and reacted for 1.5 hours; after the reaction was completed, the above mixture was poured into cyclohexane, and the precipitate was repeatedly washed with water and dried to obtain vinylated gatifloxacin;
[0033] (2) N -Reversible addition-fragmentation chain transfer polymerization of isopropylacrylamide: 2 parts of 2-cyanopropyl-2-ylbenzodisulfide, N - Mix 140 parts of isopropylacrylamide, 0.14 parts of azobisisoheptanonitrile and 180 parts of 1,4-dioxane evenly, seal, cycle freezing-thawing and deoxygenation, and then raise the temperature to React at 60°C for 6 hours, when the reaction is over, pour the above mixture into n-hexane, and dry the precipita...
Embodiment 3
[0039] (1) Norfloxacin vinylation: 20 parts of norfloxacin, N ,N - Mix 8 parts of diisopropylethylamine and 500 parts of chloroform evenly, stir at 5°C for 60 minutes, then add 8 parts of hex-5-enoyl chloride dropwise under continuous stirring and nitrogen protection, and raise the temperature to 35°C after the dropwise addition React for 2.5 hours; after the reaction is completed, pour the above mixture into n-heptane, and the precipitate is repeatedly washed with water and dried to obtain vinylated norfloxacin;
[0040] (2) N -Reversible addition-fragmentation chain transfer polymerization of isopropylacrylamide: 4 parts of 2-cyano-2-propyl dodecyl trithiocarbonate, N - Mix 300 parts of isopropylacrylamide, 0.24 parts of azobicyclohexanenitrile and 400 parts of pyridine evenly, seal, cycle freezing-thawing and deoxygenation, then raise the temperature to 65°C for 12 hours under continuous stirring and nitrogen protection, After the reaction, the above mixture is poured ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com