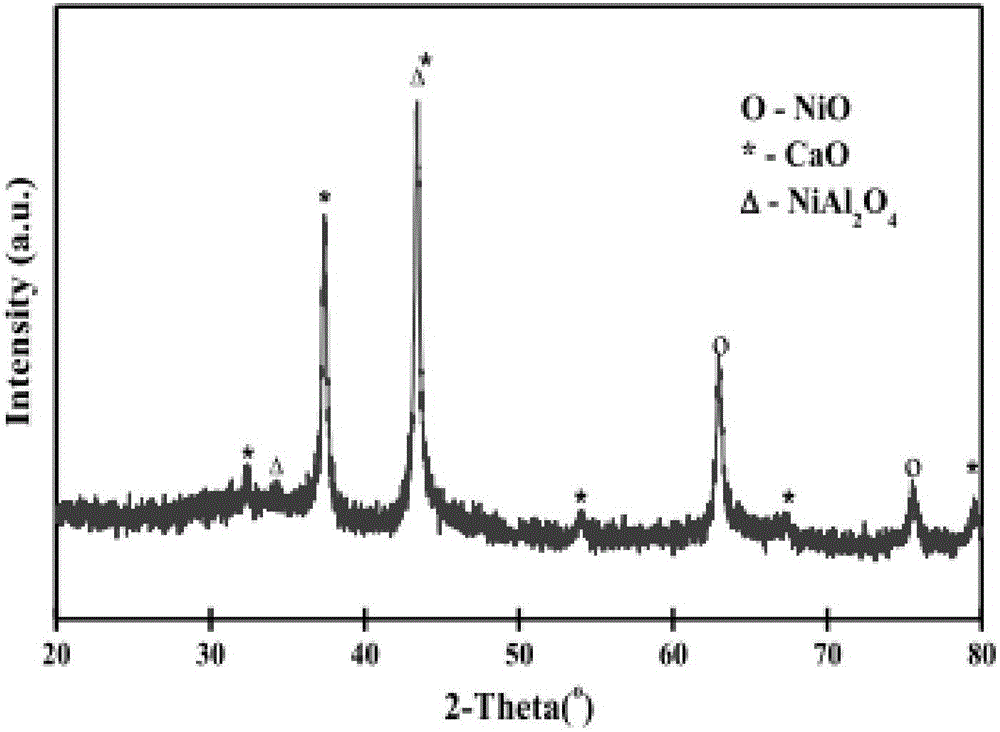
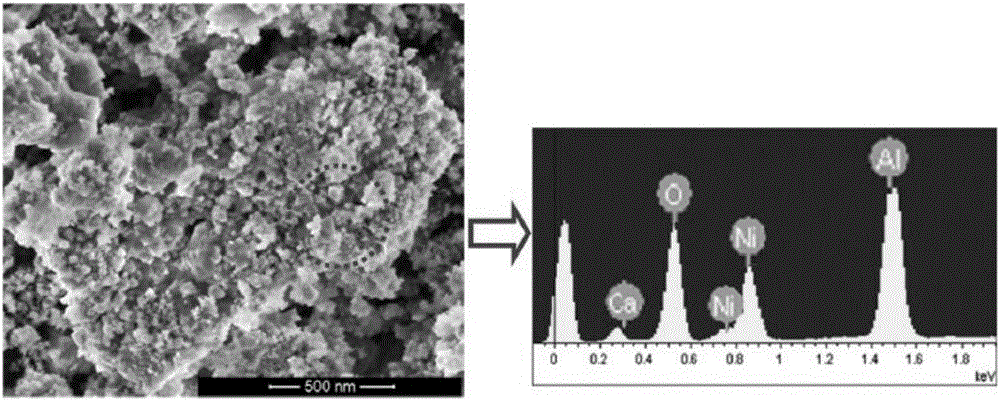
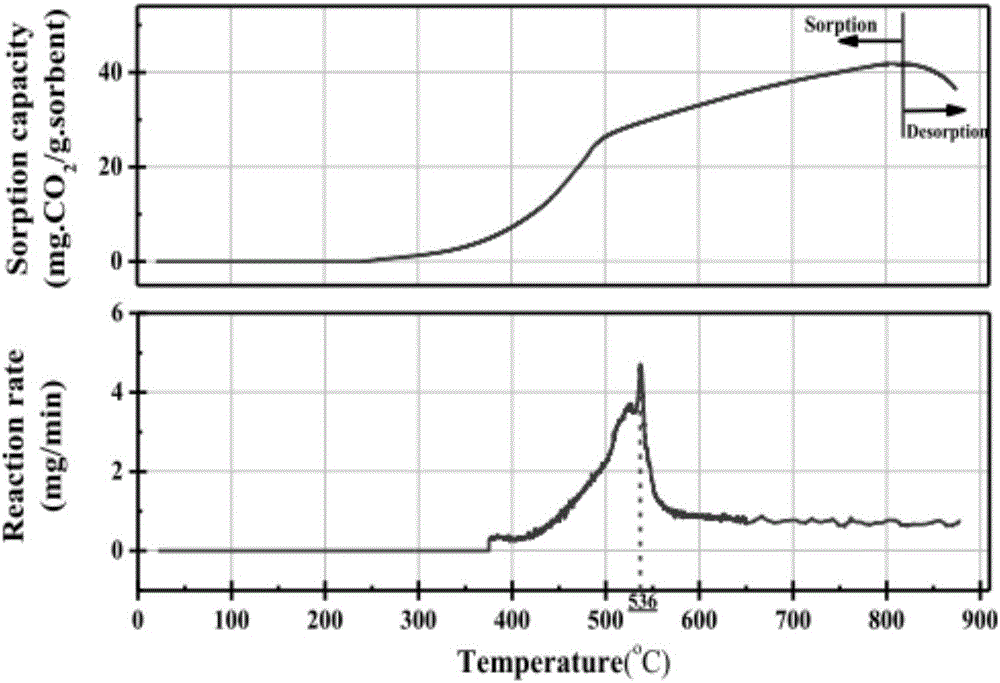
Multi-functional catalyst for reforming hydrogen production and preparation method and application of catalyst
A technology of reforming hydrogen production and composite functions, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, heterogeneous catalyst chemical elements, etc., can solve the problem of increasing process complexity and unfavorable regeneration process Operation, it is difficult to ensure the uniformity and stability of mixing, etc., to achieve the effects of avoiding rapid deactivation, good CO2 adsorption performance, and good catalytic hydrogen production performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Weigh 3.5g analytically pure Ca(NO 3 ) 2 ·6H 2 O, use deionized water to prepare a calcium nitrate aqueous solution with a mass concentration of 40%, weigh 3g of γ-Al through a 70 mesh sieve 2 o 3 Stir and mix evenly with calcium nitrate aqueous solution at 53°C for 3.5 hours; add 1 mol / L ammonia water dropwise to adjust the pH of the mixture to 8.5, continue stirring for 4 hours, and let stand for 1.5 hours to age.
[0036] The mixture was continuously stirred at 1 atmospheric pressure and 95°C until the water evaporated to dryness. The evaporated powder was dried in an air atmosphere at 120° C. for 24 hours, ground, and passed through a 70-mesh sieve to obtain a catalyst precursor.
[0037] Weigh 4g of analytically pure NiO, prepare a nickel nitrate solution with a mass concentration of 40% with 2.5mol / L dilute nitric acid, impregnate the catalyst precursor with nickel nitrate, stir and mix evenly at 25°C for 3.5h, and add 1mol / L dropwise ammonia water to adjust ...
Embodiment 2
[0040] Weigh 2.3g of analytically pure Ca(NO 3 ) 2 ·6H 2 O, use deionized water to prepare a calcium nitrate aqueous solution with a mass concentration of 30%, weigh 2.5g of γ-Al through a 60-mesh sieve 2 o 3 Stir and mix evenly with calcium nitrate aqueous solution at 45°C for 3 hours; add 1 mol / L ammonia water dropwise to adjust the pH of the mixture to 9.0, continue stirring for 3 hours, and let it stand for 1 hour to age.
[0041] The mixture was stirred continuously at 1 atmosphere and 90°C until the water evaporated to dryness. The evaporated powder was dried at 120° C. for 10 h in an air atmosphere, ground, and passed through a 60-mesh sieve to obtain a catalyst precursor.
[0042] Weigh 4.2g of analytically pure NiO, prepare a nickel nitrate solution with a mass concentration of 30% with 2.5mol / L dilute nitric acid, impregnate the catalyst precursor with nickel nitrate, stir and mix at 10°C for 3 hours, and add 1mol / L dropwise ammonia water to adjust the pH of the...
Embodiment 3
[0045] Weigh 5.2g analytically pure Ca(NO 3 ) 2 ·6H 2 O, use deionized water to prepare a calcium nitrate aqueous solution with a mass concentration of 50%, and weigh 3.5g of γ-Al through a 60-mesh sieve 2 o 3 Stir and mix evenly with calcium nitrate aqueous solution at 65°C for 4 hours; add 1 mol / L ammonia water dropwise to adjust the pH of the mixture to 9.5, continue stirring for 5 hours, and let stand for 2 hours to age.
[0046] The mixture was continuously stirred under the conditions of 1 atmosphere pressure and 93°C until the water evaporated to dryness. The evaporated powder was dried at 120° C. for 10 h in an air atmosphere, ground, and passed through a 60-mesh sieve to obtain a catalyst precursor.
[0047] Weigh 2.3g of analytically pure NiO, prepare a nickel nitrate solution with a mass concentration of 50% with 2.5mol / L dilute nitric acid, impregnate the catalyst precursor with nickel nitrate, stir and mix at 30°C for 3 hours, and add 1mol / L dropwise ammonia ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com