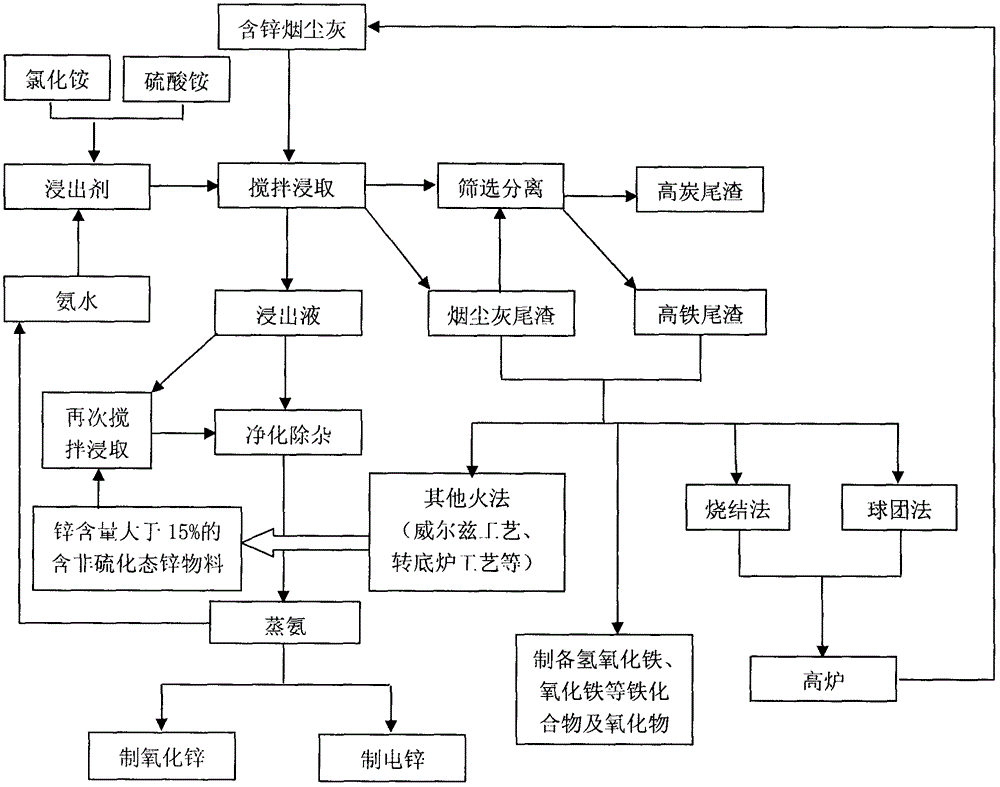
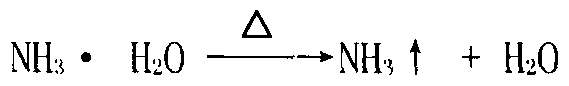Utilizing method of zinc-containing dust ash of steel plant
A steel plant, smoke and dust technology, applied in the field of utilization of zinc-containing soot and dust in steel plants, can solve the problems of large consumption of leaching agent, low recovery rate, and the lack of a complete chain of iron and carbon resources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Raw material: soot ash 1# from a steel factory in Sichuan, its composition is calculated by mass percentage (%):
[0066] Zn12.7% Fe37.14% Pb0.85% Cd0.007% C28% Alkali metal (k, Na) 2.9%
[0067] Method for preparing electric zinc and recovering iron carbon smelting:
[0068] (1) Leaching: the soot ash 1 # Use 15L ammonia water-ammonium chloride solution as leaching agent to carry out stirring leaching for many times, control liquid-solid mass ratio 4: 1; Wherein, the molar concentration c(NH 3 ·H 2 O)=4.5mol / L, [NH 3 ]: [NH 4 + ]=2:1, add sodium hypochlorite in the amount of adding 0.1kg sodium hypochlorite in every cubic meter of leaching agent; when leaching, adopt ball mill, and guarantee that the leaching time in the ball mill is 80 minutes, the ball mill outlet material all passes through 100 mesh sieve; The leaching time is 2 hours and the temperature is 40°C; the soot and ash tailings obtained from the first leaching are leached again with the above-mentio...
Embodiment 2
[0074] Raw material: soot ash 2# from a steel factory in the south, the mass percentage (%) of its composition is:
[0075] Zn7.2% Fe29.6% Pb0.87% C15.24% Si8.7%
[0076] Method for preparing zinc oxide and recycling iron carbon:
[0077] (1) Leaching: the soot ash 2 # Use 150L ammonia water-ammonium sulfate solution as leaching agent to carry out stirring leaching for many times, control liquid-solid mass ratio 5: 1; Wherein, the molar concentration of total ammonia in the leaching agent [NH 3 ]=7mol / L, [NH 3 ]: [NH 4 + ]=3:1; during leaching, ball milling is adopted, and the leaching time in the ball mill is guaranteed to be 60 minutes, the materials at the outlet of the ball mill all pass through a 120 mesh sieve, the leaching time is 1.5 hours each time, and the temperature is 20°C; The soot ash tailings were leached again with the above-mentioned leaching agent, and the final leaching residue after washing with water contained Zn=0.59%, and the leaching rate of zinc ...
Embodiment 3
[0085] Raw material: soot ash 3# from a steel factory in Southwest China, its composition is calculated by mass percentage:
[0086] Zn 15.4% Fe32.53% Pb0.67% C25.28% Si 8.67%
[0087] Method for preparing zinc oxide and recovering iron carbon smelting:
[0088] (1) Leaching: the soot ash 3 after stirring and activation # Use 300L ammonia water-ammonium chloride+ammonium sulfate solution (70% ammonium chloride+30% ammonium sulfate) as the leaching agent to carry out stirring and leaching for many times, and control the liquid-solid mass ratio to 4:1; wherein, the total ammonia in the leaching agent The molar concentration of [NH 3 ]=5.8mol / L, [NH 3 ]: [NH 4 + ]=2:1, add hydrogen peroxide in the amount of adding 1kg of hydrogen peroxide in every cubic meter of leaching agent; when leaching, adopt ball mill, and ensure that the leaching time in the ball mill is 80 minutes, and the materials at the outlet of the ball mill all pass through a 100-mesh sieve; The leaching time...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com