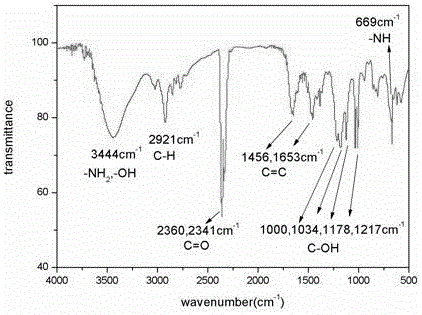
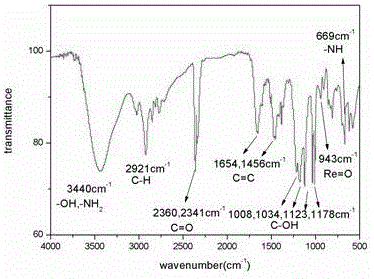
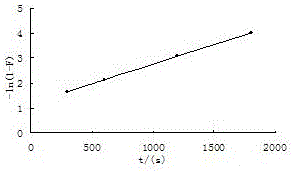Method for recycling associated rhenium resources from sandstone-type uranium deposit in-situ leaching uranium exploration process adsorption tail liquid
An in-situ leaching technology for mining uranium and sandstone, applied in the field of recovery of associated rhenium resources, can solve the problems of difficult preparation of high-purity ammonium perrhenate, low adsorption selectivity, complex synthesis process, etc., and achieve obvious social and economic benefits, The effect of high product purity and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The sandstone-type uranium ore containing associated rhenium resources is added to the 15g / L dilute sulfuric acid solution according to the ratio of uranium ore: leachate (volume ratio) = 1:10, 1g / L is added, and after 18 hours of shaking and soaking at room temperature, After filtration, an acidic mixed solution of uranium and rhenium is obtained. The slag is washed three times with a small amount of leaching solution, and the washing water is incorporated into the leaching solution to obtain an acidic mixed solution of uranium and rhenium and filter residue (the leaching rate of uranium in slag is 90%, the leaching rate of rhenium is 80%, pH=1.77 .Eh=533mv). Using D263 anion exchange resin as the adsorbent, using the saturated re-adsorption process, the uranium and rhenium in the leaching solution are adsorbed, and 6% ammonium nitrate (NH 4 NO 3 ) after desorbing uranium, the resin returns to the adsorption process after transformation. After a certain cycle period...
Embodiment 2
[0048] The sandstone-type uranium ore containing associated rhenium resources is added to the 20g / L dilute sulfuric acid solution according to the ratio of uranium ore: leachate (volume ratio) = 1:12, 1.5g / L is added, and after soaking at room temperature for 20 hours , after filtration, the acidic mixed solution of uranium and rhenium was obtained. The slag is washed three times with a small amount of leaching solution, and the washing water is incorporated into the leaching solution to obtain an acidic mixed solution of uranium and rhenium and filter residue (the leaching rate of uranium in slag is 92%, the leaching rate of rhenium is 84%, pH=1.68 .Eh=541mv). Using D263 anion exchange resin as the adsorbent, using the saturated re-adsorption process, the uranium and rhenium in the leaching solution are adsorbed, and 6% ammonium nitrate (NH 4 NO 3 ) after desorbing uranium, the resin returns to the adsorption process after transformation. After a certain cycle period, the ...
Embodiment 3
[0050] The sandstone-type uranium ore containing associated rhenium resources is added to the 20g / L dilute sulfuric acid solution according to the ratio of uranium ore: leachate (volume ratio) = 1:8, 2g / L is added, and after 16 hours of shaking and soaking at normal temperature, After filtration, an acidic mixed solution of uranium and rhenium is obtained. The slag is washed three times with a small amount of leaching solution, and the washing water is incorporated into the leaching solution to obtain an acidic mixed solution of uranium and rhenium and filter residue (the leaching rate of uranium in slag is 91%, the leaching rate of rhenium is 83%, pH=1.69 .Eh=535mv). Using D263 anion exchange resin as the adsorbent, using the saturated re-adsorption process, the uranium and rhenium in the leaching solution are adsorbed, and 6% ammonium nitrate (NH 4 NO 3 ) after desorbing uranium, the resin returns to the adsorption process after transformation. After a certain cycle perio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com