Cloning, expression and application of alpha-L-rhamnosidase gene
A rhamnosidase and gene technology, applied in the fields of genetic engineering and enzyme engineering, can solve the problems of low yield, high cost, difficulty in separation and purification of α-L-rhamnosidase, etc., and achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
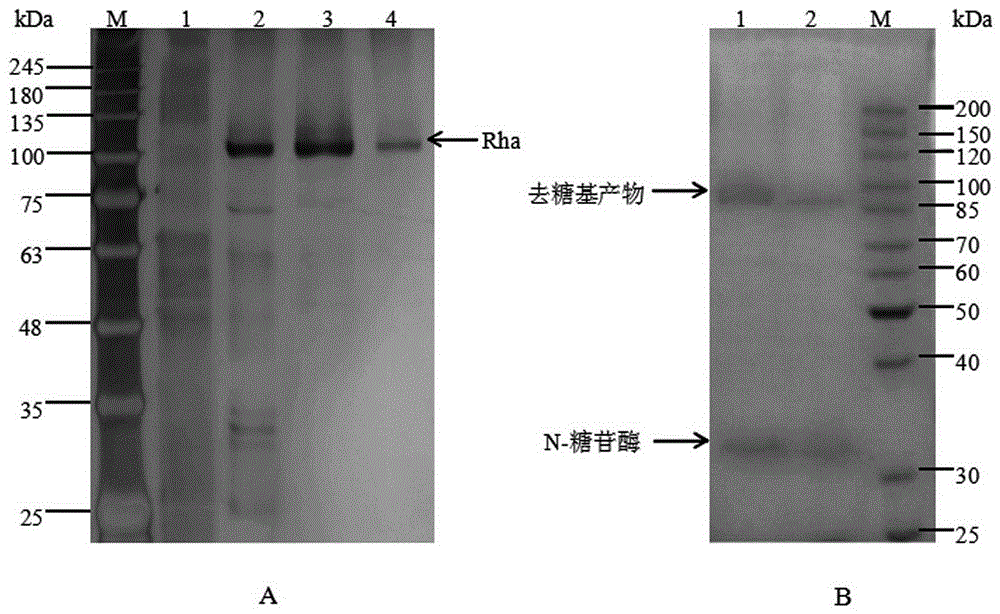
[0035] Embodiment 1, clone expression of gene
[0036] 1. Main reagents
[0037] DNA polymerase, T4 DNA ligase, DNA marker, cloning vector pMD-18T, restriction endonucleases (Bln Ⅰ and Not Ⅰ), competent cell preparation kits were purchased from Takara Company; PCR product recovery kit, gel recovery Kits, rapid plasmid extraction kits, etc. were purchased from Shanghai Sangong; RNA extraction kits, cDNA reverse transcription kits were purchased from Beijing Quanshijin. RCR primers were synthesized by Beijing Liuhe Huada Gene Company; IPTG, ampicillin, and sodium dodecyl sulfate (SDS) were purchased from Shanghai Sangong; other routine reagents were chemically analytically pure.
[0038] 2. Plasmids and strains
[0039] Aspergillus tubingensis, Escherichia coli DH5α, Pichia pastoris GS115 and pPIC9K are all conventional biological materials.
[0040] 3. Method
[0041] 3.1 Aspergillus tubingensis was inoculated on the activation medium, cultured at 28°C for 2 days, transferr...
Embodiment 2
[0053] Example 2. Enzymatic properties of recombinant α-L-rhamnosidase
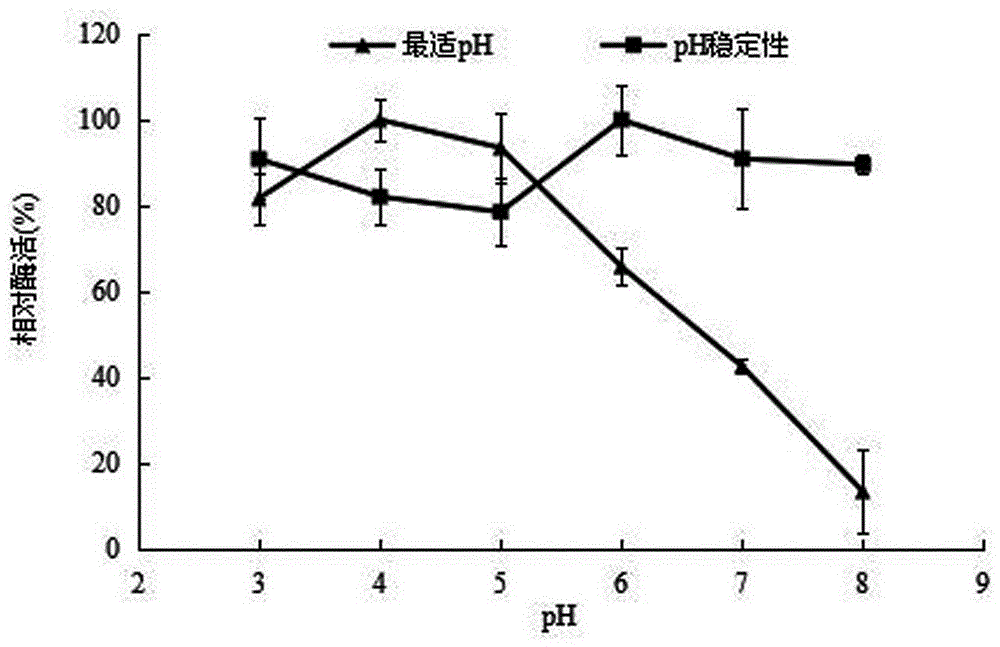
[0054] 2.1 The influence of pH on recombinant α-L-rhamnosidase
[0055] Na at pH 3.0, 4.0, 5.0, 6.0, 7.0 and 8.0 at a concentration of 0.05 mol / L 2 HPO 4 -C 4 h 2 o 7 Enzyme activity was measured in the reaction system, and the highest enzyme activity was taken as 100%, and the rest were compared with it, and the relative enzyme activity was plotted against the pH to obtain the enzyme reaction pH impact on enzyme activity; Glycosidase was mixed with 400 μL of the above-mentioned different pH buffers, and then placed at 4°C for 24 hours to measure the remaining enzyme activity, and the relative enzyme activity was plotted against the pH to obtain the pH stable range of the enzyme. The results are as follows figure 2 shown.
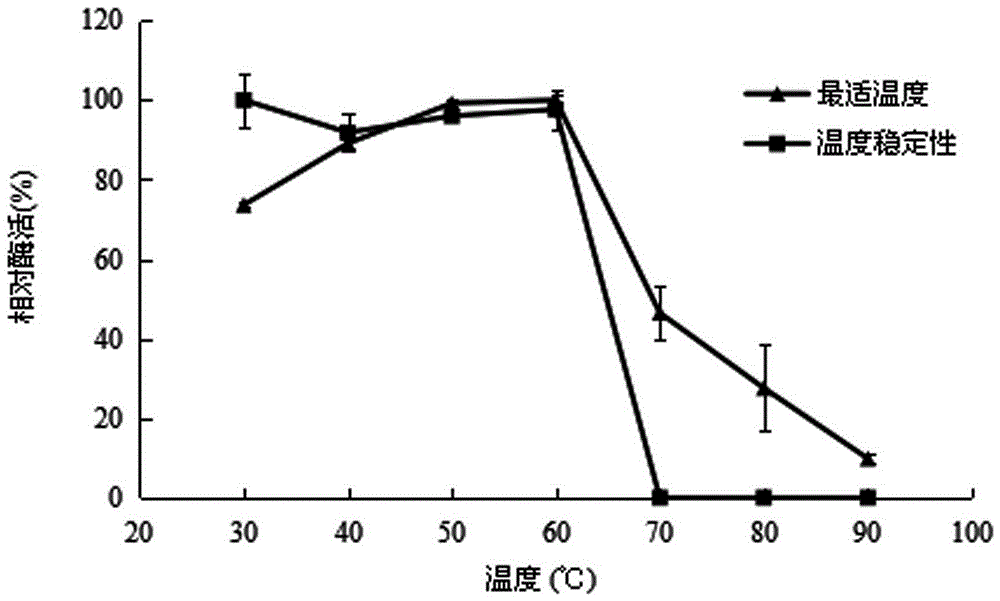
[0056] 2.2 Effect of temperature on recombinant α-L-rhamnosidase
[0057] The enzyme activity was measured at 30, 40, 50, 60, 70, and 80°C respectively, and the enzyme activity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com