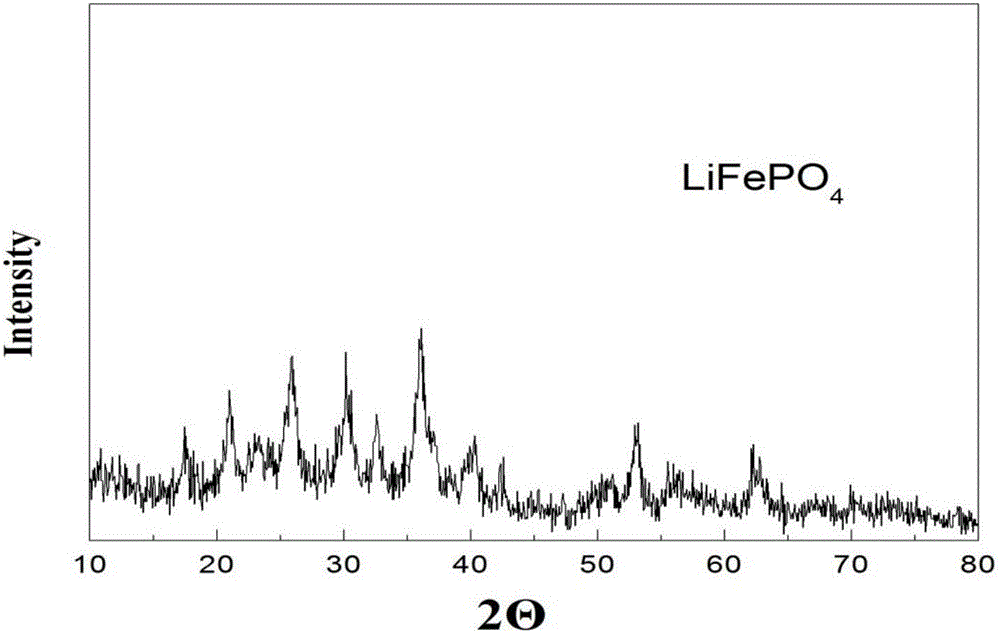
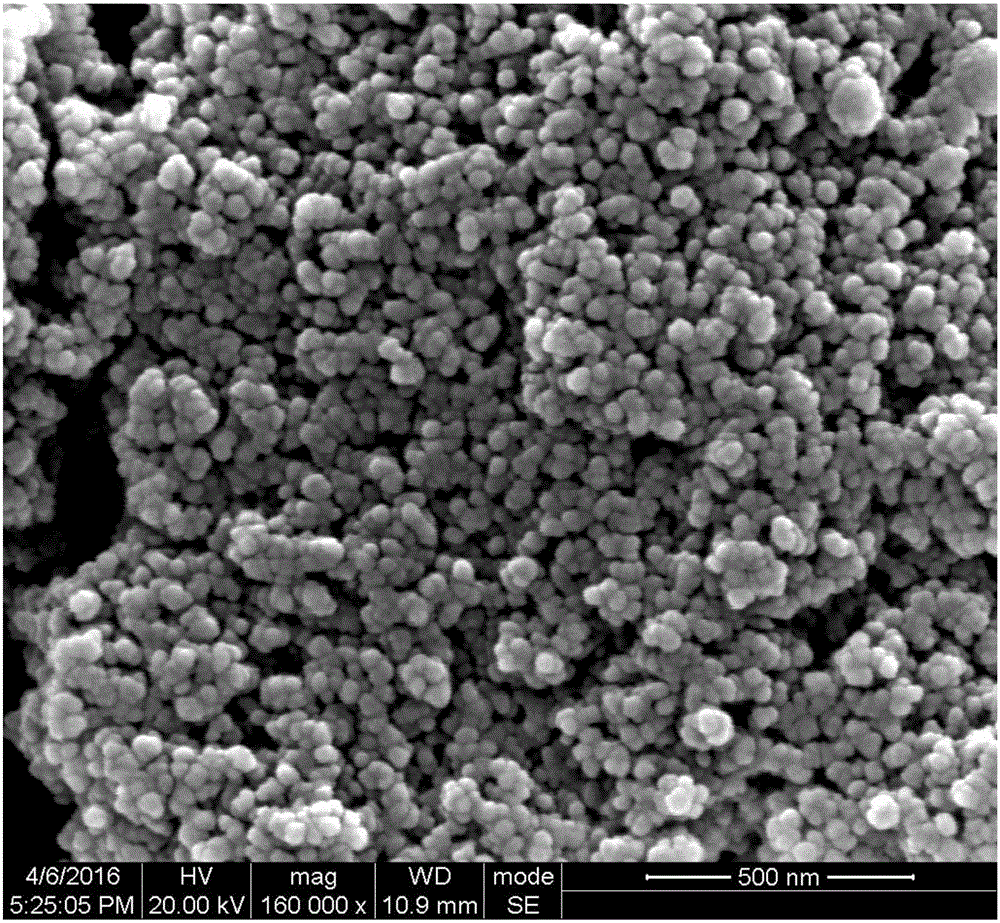
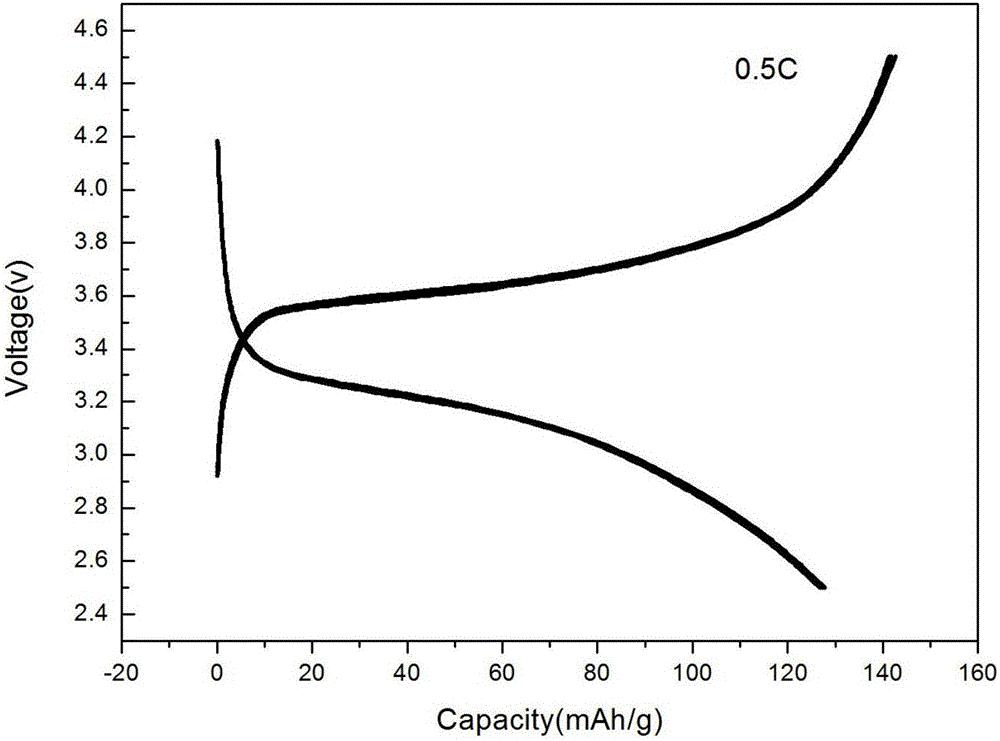
Fluorine-doped carbon covered lithium iron phosphate, and preparation method and application thereof
A carbon-coated lithium iron phosphate and lithium iron phosphate technology, which is applied in the field of energy storage materials and electrochemistry, can solve the problem of low specific capacity, poor high-rate performance, pure-phase lithium iron phosphate electronic conductivity, and lithium ion diffusion rate. Low-level problems, to achieve the effect of improved material properties, good repeatability and stability, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of nano-scale pure phase lithium iron phosphate by hydrothermal method
[0034] A. Measure lithium hydroxide monohydrate (LiOH·H 2 O) 63g (1.5mol), phosphoric acid (H 3 PO 4 )49g (0.5mol), ferrous sulfate heptahydrate (FeSO 4 ·7H 2 (0) 139g (0.5mol), ethylene glycol 4L and ascorbic acid 28g (0.16mol);
[0035] B. Lithium hydroxide monohydrate is added to the mixed solution of phosphoric acid and ethylene glycol, after stirring for 30 minutes, add ascorbic acid and ferrous sulfate heptahydrate, stir for another 30 minutes, and then pour into the reaction kettle;
[0036] C. Put the reactor into an oven and heat it at 150°C for 10h;
[0037] D. Take out the sample, cool it down to room temperature naturally, then pour it into a test tube and centrifuge at 9000r / min for 10min;
[0038] E. Pour off the upper layer solution in the test tube, wash the sample with ethanol solution and deionized water in turn (3 times each), centrifuge until the upper laye...
Embodiment 2
[0049] (1) Preparation of nano-scale pure phase lithium iron phosphate by hydrothermal method
[0050] A. Measure lithium chloride 63g (1.5mol), phosphoric acid 49g (0.5mol), ferrous chloride 139g (0.5mol), water 4L and ascorbic acid 28g (0.16mol);
[0051] B. Add lithium chloride to the solution of phosphoric acid and water, add ascorbic acid and ferrous chloride after stirring for 30min, stir for another 30min, then pour into the reaction kettle;
[0052] C. Put the reactor into an oven and heat at 180°C for 5 hours;
[0053] D. Take out the sample, cool it to room temperature naturally, then pour it into a test tube and centrifuge at 8000r / min for 20min;
[0054] E. Pour off the upper layer solution in the test tube, wash the sample with ethanol solution and deionized water in turn (3 times each), centrifuge until the upper layer solution is clear, and then put it in a vacuum oven for drying at 60°C to obtain nano-scale pure phase Lithium iron phosphate powder;
[0055] ...
Embodiment 3
[0059] (1) Preparation of nano-scale pure phase lithium iron phosphate by hydrothermal method
[0060] A. Measure lithium chloride 1.27kg, phosphoric acid 0.98kg, ferrous chloride tetrahydrate 1.99kg, glycerol 0.6L and ascorbic acid 0.14kg;
[0061] B. Lithium chloride is added in the mixed solution of phosphoric acid and glycerol, after stirring for 30min, add ascorbic acid and ferrous chloride, stir for another 30min, then pour into the reactor;
[0062] C. Put the reactor into an oven and heat it at 190°C for 4 hours;
[0063] D. Take out the sample, cool it to room temperature naturally, then pour it into a test tube and centrifuge at 8000r / min for 20min;
[0064] E. Pour off the upper layer solution in the test tube, wash the sample with ethanol solution and deionized water in turn (3 times each), centrifuge until the upper layer solution is clear, and then put it in a vacuum oven for drying at 60°C to obtain nano-scale pure phase Lithium iron phosphate powder;
[0065...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com