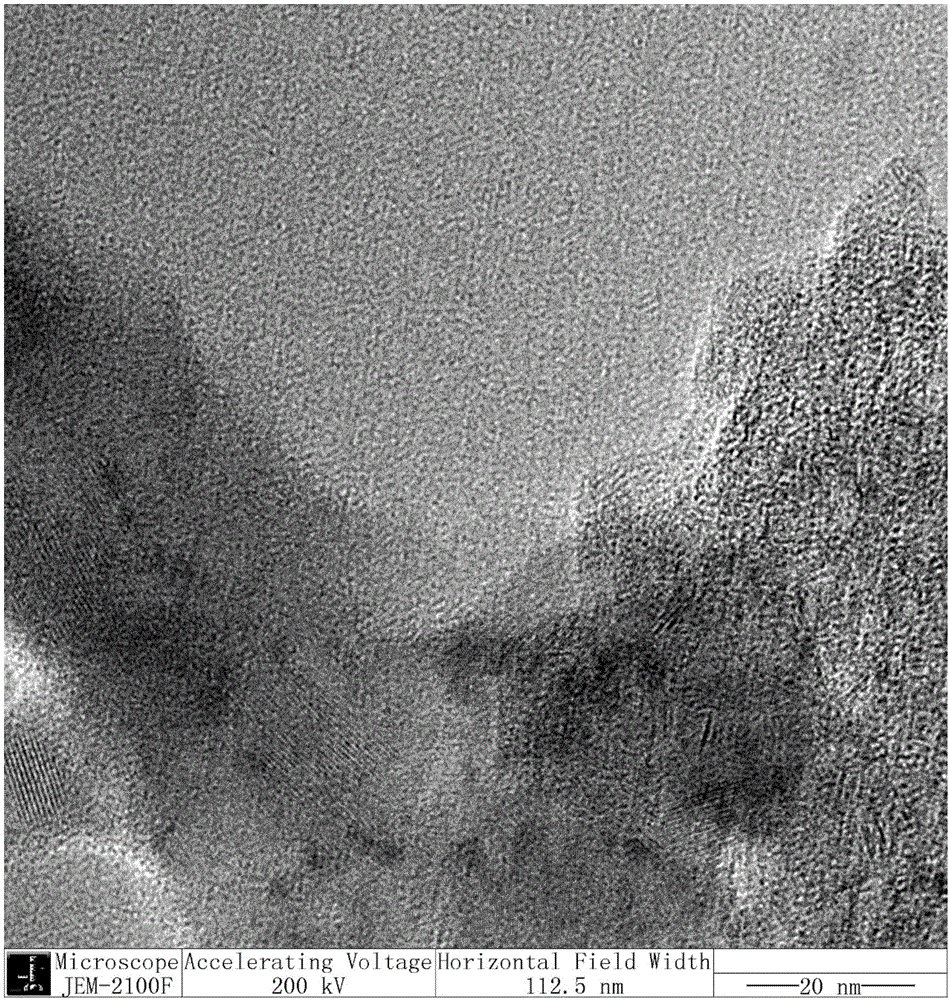
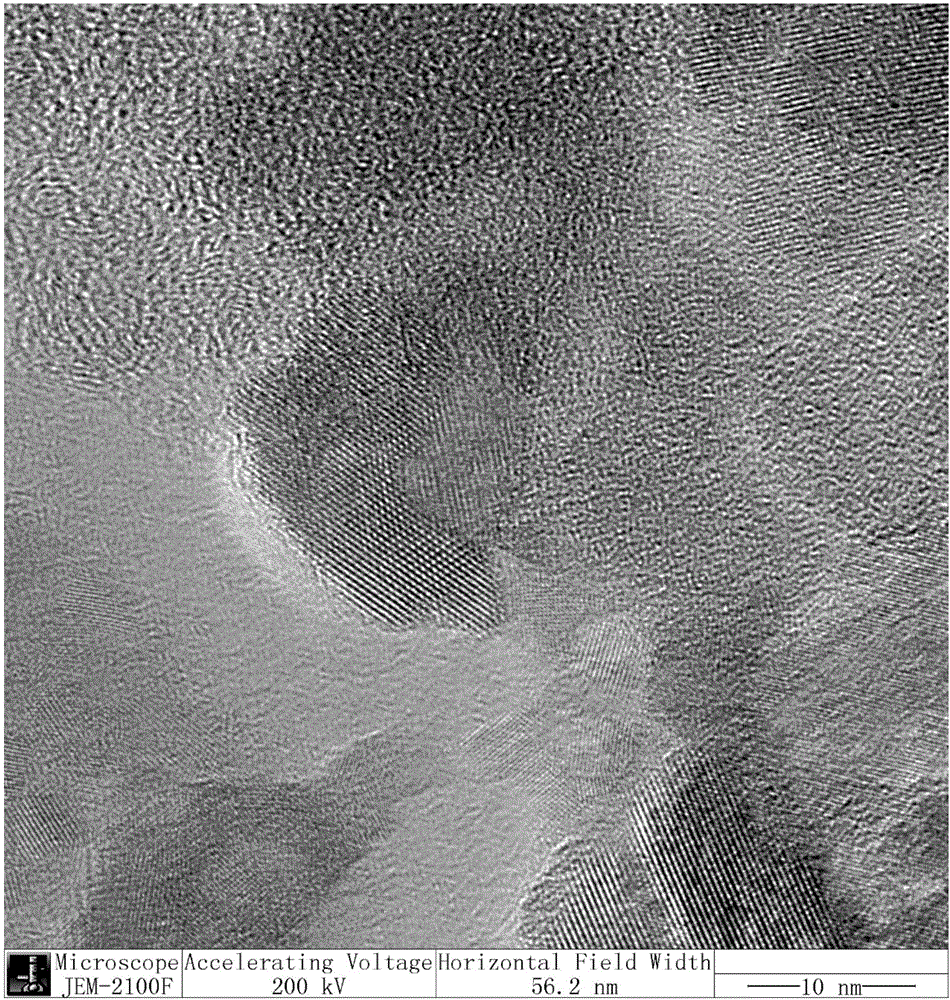
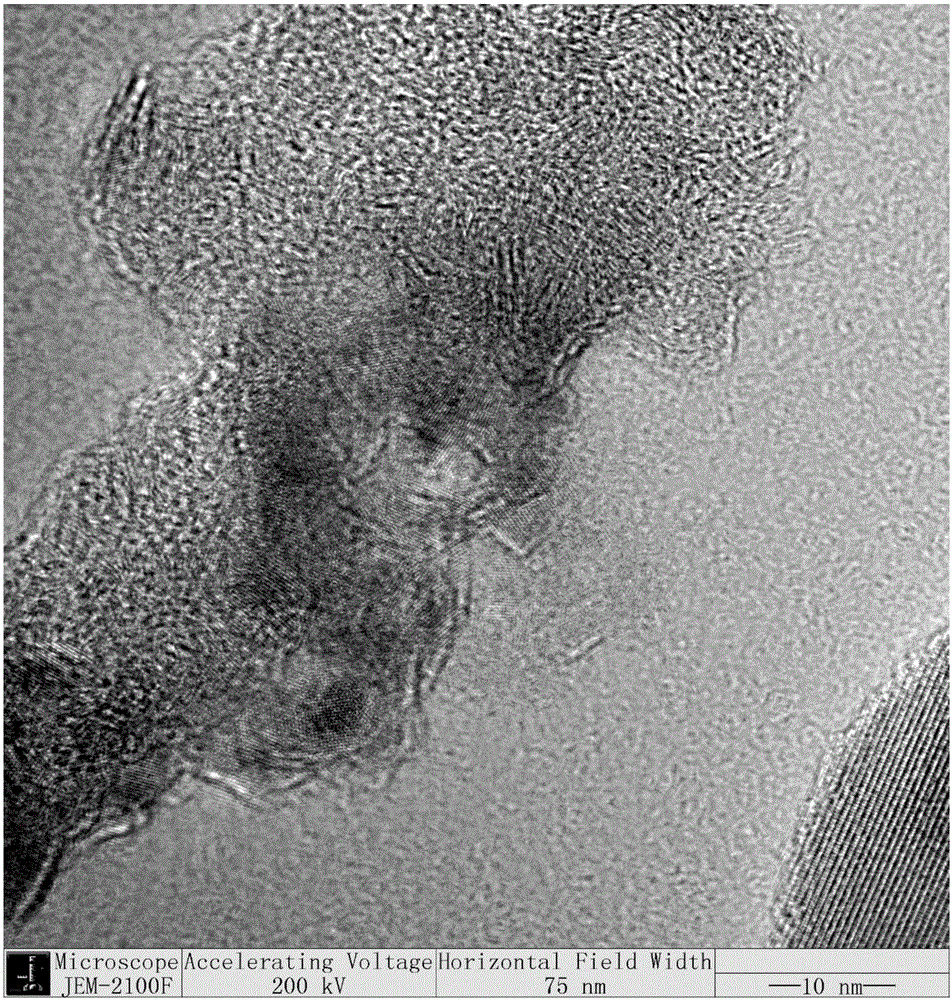
Synthesis of molybdenum sulphide with controllable layer number and application of molybdenum sulphide with controllable layer number in aromatic phenol and ether reactions
A technology of molybdenum sulfide and aromatic phenol, applied in the field of catalytic conversion of biomass derivatives, can solve the problems of high cost and low yield, and achieve the effects of high conversion rate and yield, simple and easy preparation method and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of catalyst precursor: Dissolve 2.0g molybdenum hexacarbonyl and 4.5g tetraethylthiuram disulfide in a molar ratio of 1:2 in 60ml of acetone, heat to 60°C under an argon atmosphere, and reflux for 3 hours to form a purple color Precipitation, filtered by suction, washed with pentane to obtain a purple precipitate, and dried in an oven at 120°C for 12 hours to obtain MoS 2 Precursor Mo(dedtc) 4 .
[0028] Table 1. Catalyst precursor thermogravimetric analysis data
[0029]
[0030]
[0031] From the thermogravimetric results, it can be seen that the actual precursor P has a residual mass of 23.4 ≈ 23.5 (theoretical mass residual).
Embodiment 2
[0033] MoS 2 Preparation of / AC catalyst: the catalyst precursor prepared in Example 1 was mixed with 10wt% MoS 2 / AC conversion Dissolved in N,N-dimethylformamide, completely dissolved and impregnated on the carrier AC for 24 hours, and then dried at 120°C for 12 hours; the obtained catalyst was transferred to a tube furnace for temperature programming Pyrolysis, the specific reaction process is: load 0.5-4g of catalyst in the constant temperature zone of a quartz reaction tube with an inner diameter of 1.8cm, raise the temperature from room temperature 10°C / min to 320°C, and then keep it for 4h, and the flow rate of argon gas is 60ml / min. MoS 2 MoS with a loading of 10 wt% 2 / AC catalyst, denoted as MoS 2 / AC-320°C.
[0034] Other conditions remain the same, and only changing the decomposition temperature of the catalyst precursor can obtain catalysts with different crystallinity and different layers, which are respectively denoted as MoS 2 / AC-400℃, MoS2 / AC-600℃, MoS ...
Embodiment 3
[0036] MoS 2 / γ-Al 2 o 3 Preparation of the catalyst: the preparation process is similar to Example 2, the difference is that the support is replaced by γ-Al 2 o 3 , get MoS 2 MoS with a loading of 10wt% 2 / γ-Al 2 o 3 catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com