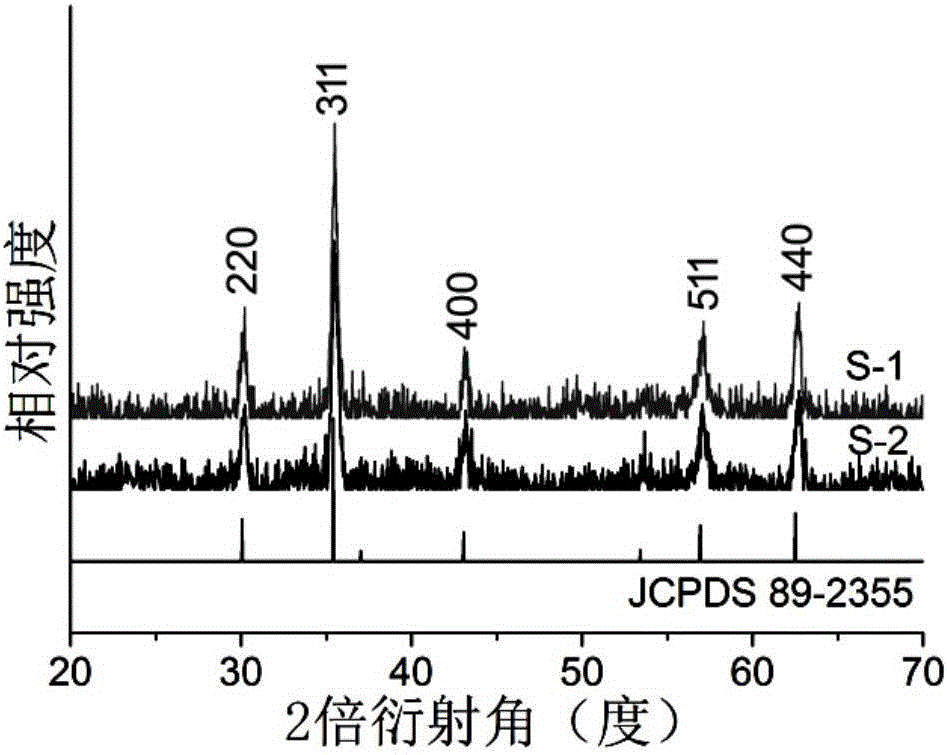
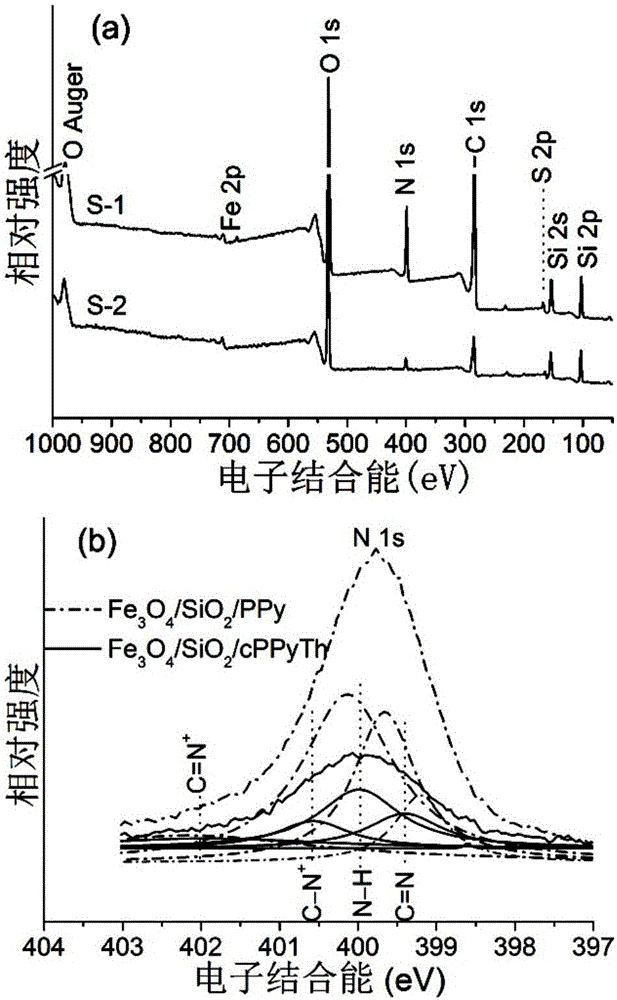
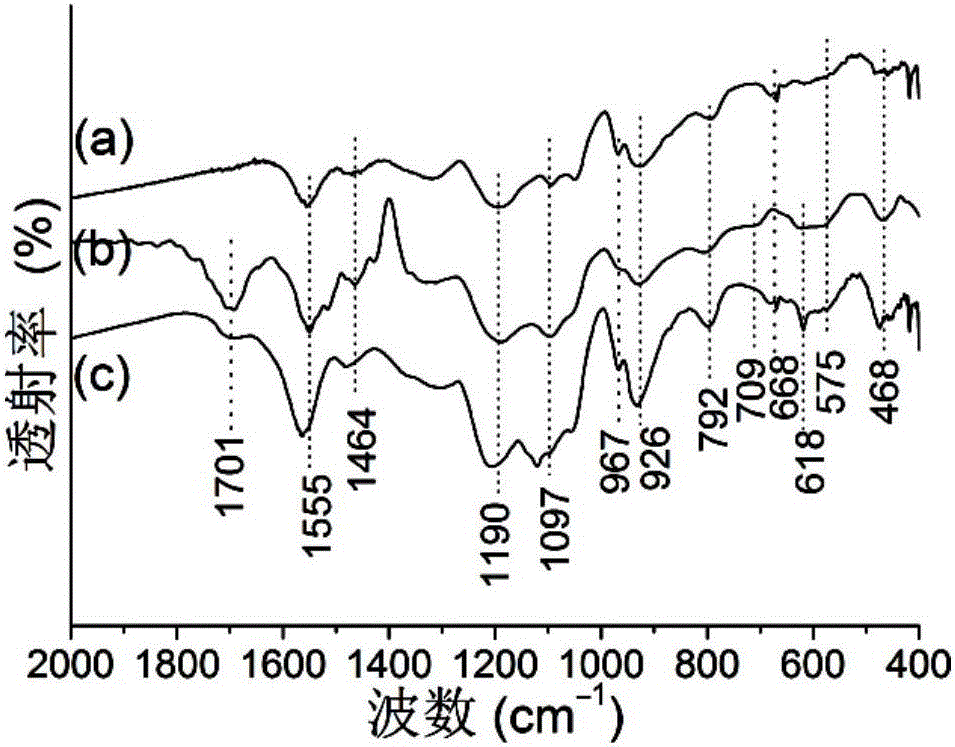
Selective separation method of gold ions
A separation method and technology for gold ions, applied in chemical instruments and methods, alkali metal compounds, improvement of process efficiency, etc., can solve the problems of cumbersome synthesis steps of adsorbent materials, poor acid resistance, incomplete conductive polymers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] a kind of Fe 3 o 4 / SiO 2 The preparation method of / cPPyTh magnetic nanocomposite, the steps are as follows:
[0061] (1) Under the protection of nitrogen, add 9mmol ferric chloride hexahydrate, 5mmol ferrous sulfate heptahydrate and 120mL distilled water into a 250mL four-necked bottle, stir and dissolve at room temperature, raise the temperature of the solution to 50°C, and then add 3.3mL Concentrated ammonia water (28wt%), after the addition, the temperature of the solution was raised to 80°C, stirred for 1 hour, and cooled to room temperature to obtain solution A;
[0062] (2) Add 1.7mL tetraethyl orthosilicate to solution A, stir at room temperature for 4 hours; magnetically separate, keep the solid, wash the solid repeatedly with distilled water until the supernatant is neutral, magnetically separate, keep the solid, and place the solid in Dry in a vacuum oven at 40°C for 6 hours to obtain Fe 3 o 4 / SiO 2 ;
[0063] (3) Under nitrogen protection, add 0.6g ...
Embodiment 2
[0067] a kind of Fe 3 o 4 / SiO 2 The preparation method of / cPPyTh magnetic nanocomposite, the steps are as follows:
[0068] (1) Under nitrogen protection, add 10mmol ferric nitrate nonahydrate, 5mmol ferrous sulfate heptahydrate and 67mL distilled water into a 250mL four-necked bottle, stir and dissolve at room temperature, raise the solution temperature to 60°C, then add 5mL concentrated ammonia water ( 28wt%), after the addition, the temperature of the solution was raised to 90°C, stirred for 0.5h, and cooled to room temperature to obtain solution A;
[0069] (2) Add 1.9mL methyl orthosilicate to solution A, stir at room temperature for 2 hours; magnetically separate, keep the solid, wash the solid repeatedly with distilled water until the supernatant is neutral, magnetically separate, keep the solid, and place the solid in Dry in a vacuum oven at 60°C for 4 hours to obtain Fe 3 o 4 / SiO 2 ;
[0070] (3) Under nitrogen protection, add 0.6g Fe to a 250mL four-necked ...
Embodiment 3
[0080] A magnetic nanocomposite is used for the selective separation of gold ions, the steps are as follows:
[0081] (1) Take an appropriate amount of standard solutions of gold (III), magnesium (II), copper (II), zinc (II), arsenic (V), cadmium (II) and lead (II) respectively, mix them together, Prepare a mixed solution with a pH of 5 whose ion concentration is 90 mg / L, and divide the above mixed solution into 10 mL aliquots.
[0082] (2) Add 1, 2, 4, 8, 12, and 16 mg of magnetic nanocomposite sample S-1 to each equal portion of the mixed solution, and disperse evenly with ultrasonic waves, then place it in an oscillator and oscillate at room temperature for 2 hours. 100r / min, magnetic separation, the supernatant was taken for ICP-OES detection, and the adsorption amount of various ions was calculated.
[0083] (3) The magnetic adsorbent recovered after the adsorption separation is respectively placed in the mixed eluent of hydrochloric acid-thiourea (hydrochloric acid 5wt%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Remanence | aaaaa | aaaaa |
| Coercivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com