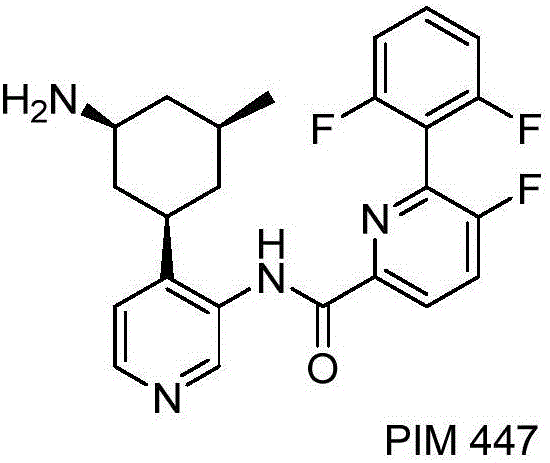
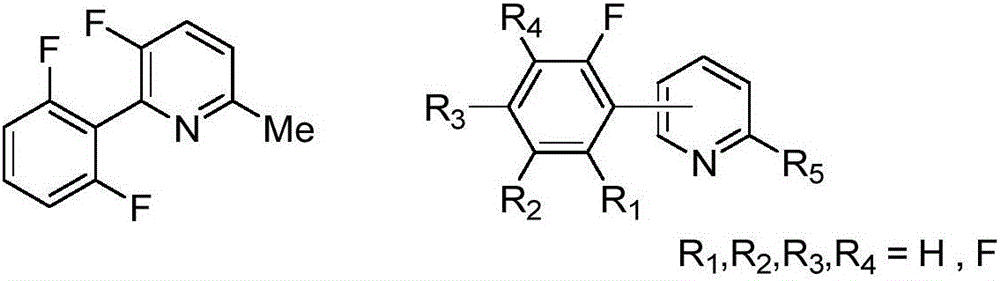
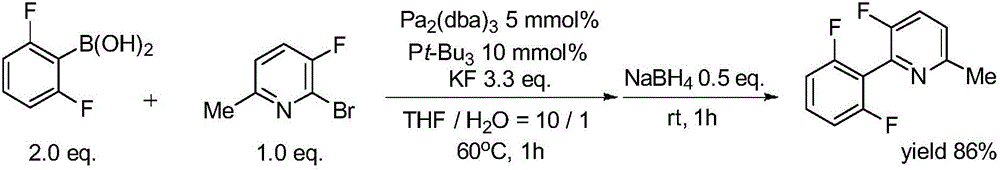Synthesis method of (poly)fluorophenylpyridine compounds
A technology of polyfluorophenylpyridine and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of expensive catalysts, expensive heavy metals, and high comprehensive costs, and is conducive to the promotion of large-scale industrial production, the value of large-scale industrial production, and the reduction of post-processing effect of difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of 2-(2′,6′-difluorophenyl)-3-fluoro-6-methylpyridine with the following structural formula
[0039]
[0040] 1. Under anhydrous and oxygen-free conditions, dissolve 3.42g (30mmol) 1,3-difluorobenzene in 10mL tetrahydrofuran, then slowly add 6mL 2.5mol / L n-butyllithium hexane solution, and stir at -20°C After 4 hours, slowly add 22 mL of 1 mol / L zinc chloride solution in tetrahydrofuran, slowly raise to room temperature, and continue stirring for 2 hours.
[0041] 2. Under anhydrous and oxygen-free conditions, add 200 mg (0.8 mmol) bis(1,5-cyclooctadiene) nickel, 770 mg (1 mmol) bis(2-bis(3′,5′-bis Methyl-4'-methoxyphenyl)phosphine)phenyl ether, stirred at room temperature for 30 minutes.
[0042] 3. Under anhydrous and oxygen-free conditions, mix the reaction solutions obtained in step 1 and step 2, and add 1.90 g (10 mmol) of 2-bromo-3-fluoro-6-methylpyridine, stir at 50 ° C for 10 hours, and detect by TLC Reaction was complete, after methanol was added...
Embodiment 2
[0044] Synthesis of 2-(2',4',6'-trifluorophenyl)-3-fluoro-6-methylpyridine with the following structural formula
[0045]
[0046] 1. Under anhydrous and oxygen-free conditions, dissolve 3.17g (24mmol) 1,3,5-trifluorobenzene in 10mL tetrahydrofuran, then add 10mL 2mol / L tetrahydrofuran solution of ethylmagnesium chloride, and stir at 50°C for 18 hours , and then add 22 mL of 1 mol / L zinc chloride solution in tetrahydrofuran, and continue stirring at room temperature for 2 hours.
[0047] 2. Under anhydrous and oxygen-free conditions, add 220 mg (0.8 mmol) bis(1,5-cyclooctadiene) nickel, 801 mg (1 mmol) bis(2-bis(3′,4′-bis Methoxyphenyl)phosphine)phenyl ether, stirred at room temperature for 30 minutes.
[0048] 3. Under anhydrous and oxygen-free conditions, mix the reaction solutions obtained in step 1 and step 2, and add 1.90 g (10 mmol) of 2-bromo-3-fluoro-6-methylpyridine, stir at 50 ° C for 10 hours, and detect by TLC Reaction was complete, after methanol was added to...
Embodiment 3
[0050] Synthesis of 2-(2′,3′,5′,6′-tetrafluorophenyl)-3-fluoro-6-methylpyridine with the following structural formula
[0051]
[0052] In step 1 of Example 2, 1,3,5-trifluorobenzene was replaced with 1,2,4,5-tetrafluorobenzene whose molar weight was 0.8 times that of 1,2,4,5-tetrafluorobenzene, and ethylmagnesium chloride was replaced with an equimolar amount of isopropyl Magnesium chloride was replaced and the reaction temperature was changed to room temperature. In step 3 of Example 2, bis(2-bis(3',4'-dimethoxyphenyl)phosphine)phenyl ether was mixed with equimolar bis(2-bis(3',5'-bis Methyl-4'-methoxyphenyl) phosphine) phenyl ether replacement, other steps are the same as in Example 2, to obtain 2-(2',3',5',6'-tetrafluorophenyl)-3- Fluoro-6-picoline, its yield is 87%, and the structural characterization data is: 1 H NMR (600MHz, CDCl 3 ): δ7.46 (t, J = 8.6H, 1H), 7.22 (dd, J = 8.6, 3.8Hz, 1H), 7.19-7.13 (m, 2H), 2.61 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com