High performance liquid chromatography-time-of-flight mass spectrometry method for identifying degradation product in compound cold treatment medicine based on heart-cutting technology
A high-performance liquid chromatography and time-of-flight mass spectrometry technology, applied in the field of analytical chemistry, can solve problems such as interference and pollution, and achieve the effect of improving accuracy and reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0077] a. Preparation of ibuprofen oxidation sample: take an appropriate amount of compound cold medicine (approximately equivalent to 200 mg of ibuprofen), add 10 ml of acetonitrile, dissolve it by ultrasonic, filter, add 10 ml of 30% hydrogen peroxide, put in a water bath at 80 ° C for 1 hour, Take it out, let it cool, transfer it to a 100ml measuring bottle, make it to volume, shake it up, filter it, and wait for sample injection.
[0078] b. Preparation of oxidized samples of phenylephrine hydrochloride and chlorpheniramine maleate: take an appropriate amount of compound cold medicine (approximately equivalent to 10 mg of phenylephrine hydrochloride and 4 mg of chlorpheniramine maleate), add 20% acetonitrile -Water solvent 10ml, ultrasonic to dissolve, shake well, add 5ml of 30% hydrogen peroxide, put in 80°C water bath for 30 minutes, take it out, let cool, transfer to 25ml measuring bottle, constant volume, shake well, filter, wait for sample injection .
Embodiment 1
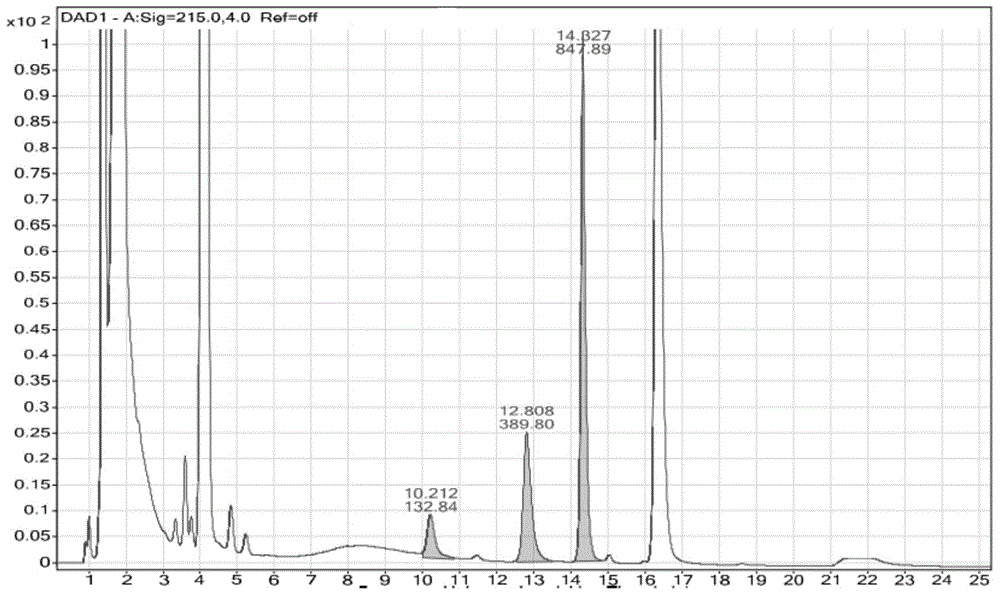
[0079] Example 1 Analysis of chlorpheniramine maleate and phenylephrine hydrochloride forced degradation products, cut as figure 1 When the middle retention time is 12.80 minutes, the instrument configuration and analysis conditions are:
[0080] One-dimensional:
[0081] High performance liquid chromatography: HPLC, Agilent 1260;
[0082] Chromatographic column: Waters spherisorb 5um SCX, 4.6*150mm;
[0083] Mobile phase A: 0.075mol / L disodium hydrogen phosphate (containing 0.1% triethylamine, adjust pH to 2.4 with phosphoric acid);
[0084] Mobile phase B: acetonitrile;
[0085] Linear Gradient:
[0086]
[0087] Detection wavelength: 215nm;
[0088] Flow rate: 1.0ml / min;
[0089] Column temperature: 35°C;
[0090] Injection volume: 20ul;
[0091] The valve switching time is 12.75-12.85min.
[0092]
[0093] Cut 1D chromatograms (eg figure 1 ) when the retention time is a component of 12.80 minutes, the two-dimensional test conditions are as follows:
[0094...
Embodiment 2
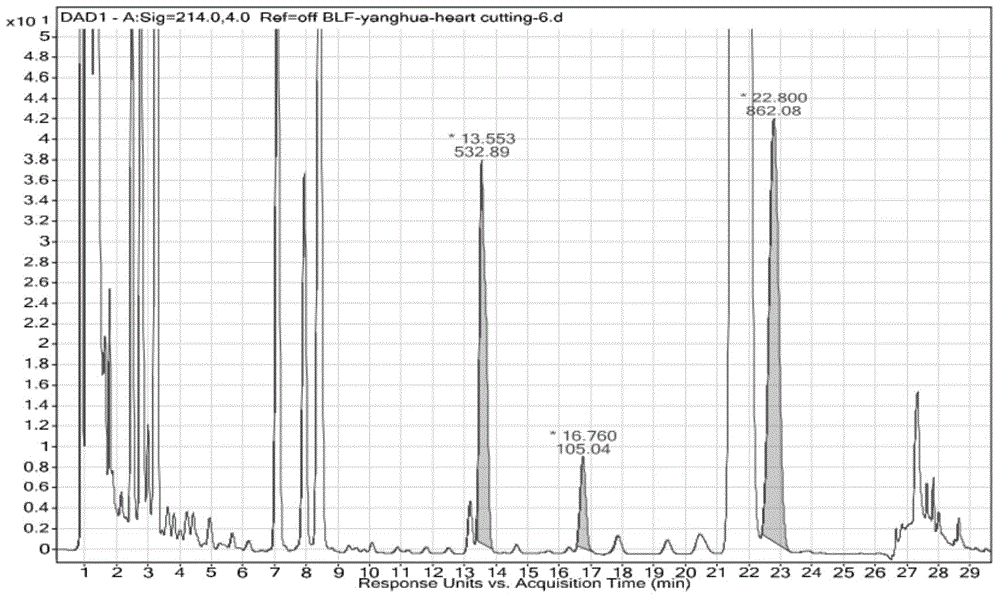
[0113] Example 2 Analysis of ibuprofen forced degradation products, cut as figure 2 When the middle component is 13.55 minutes, the instrument configuration and analysis conditions are:
[0114] One-dimensional:
[0115] High performance liquid chromatography: HPLC, Agilent 1260;
[0116] Chromatographic column: Waters BEH C18 3.5um 4.6*150mm;
[0117] Gradient elution conditions:
[0118] Mobile phase A: 0.02mol / L sodium dihydrogen phosphate (adjust the pH to 3.0 with phosphoric acid)-acetonitrile (83.5:16.5);
[0119] Mobile phase B: 0.02mol / L sodium dihydrogen phosphate (adjust the pH to 3.0 with phosphoric acid)-acetonitrile (16.5:83.5);
[0120] Linear Gradient:
[0121]
[0122] Detection wavelength: 214nm;
[0123] Flow rate: 1.4ml / min;
[0124] Column temperature: 35°C;
[0125] Injection volume: 20ul;
[0126] The valve switching time is 13.50-13.60min.
[0127]
[0128] Cut 1D chromatograms (eg figure 2 ), the two-dimensional test conditions are as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| collision energy | aaaaa | aaaaa |
| mobile phase | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com