Finasteride nano-liposome, gel and preparation method thereof
A nano-liposome and finasteride technology, applied in the field of medicine, can solve the problems of destroying the patient's skin tissue, changing the skin structure, skin damage, etc., and achieve the effect of improving poor skin permeability, good reproducibility, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
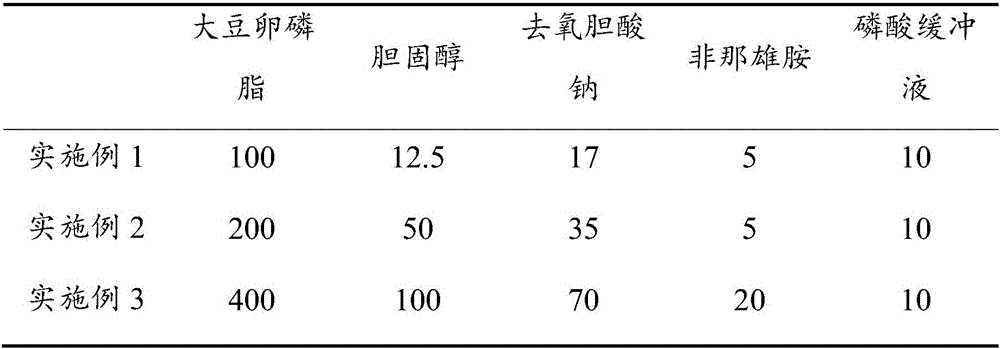
[0032] A finasteride nanoliposome, comprising the following raw materials (parts by weight) components, see Table 1:
[0033] Table 1 main drug and flexible lipid material prescription
[0034]
[0035] The preparation method is as follows:
[0036] (1) Soybean lecithin, cholesterol and finasteride are dissolved in absolute ethanol (the amount is based on the ability to dissolve the above-mentioned raw materials), and placed in an eggplant-shaped flask;
[0037] (2) Place the eggplant-shaped flask on a rotary evaporator, and remove absolute ethanol by rotary evaporation under reduced pressure in a constant temperature water bath at 37° C. to form a uniform lipid film on the wall of the bottle;
[0038] (3) Prepare a PBS solution of sodium deoxycholate, add the solution to the eggplant-shaped bottle that formed the film in step (2), in a water bath at 40°C, rotate at normal pressure, and hydrate for 60 minutes to prepare finasteride nano primary emulsion of liposomes;
[...
Embodiment 4
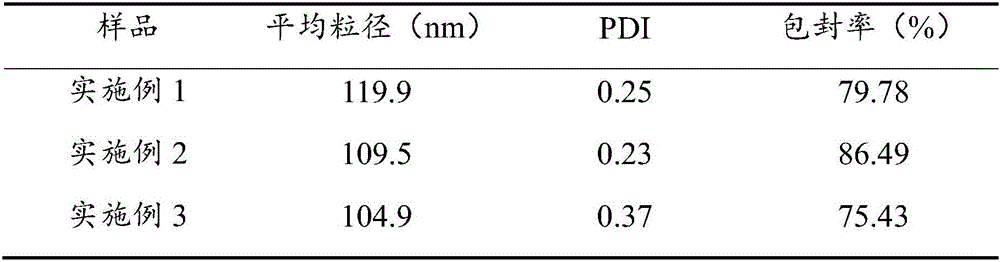
[0041] Physicochemical properties of the finasteride nanoliposomes prepared in the above examples 1-3.
[0042] The finasteride nanoliposome prepared by embodiment 1-3 is evaluated as follows:
[0043] (1) Average particle size and PDI:
[0044] Use a pipette gun to draw an appropriate amount of the flexible nanoliposome suspension of finasteride, add it to the quartz measuring cell, dilute it to 1mL with pure water, and measure the average particle size and polydispersity index of the sample with a laser scattering particle size analyzer (PDI).
[0045] (2) Determination of Encapsulation Efficiency:
[0046] The flexible nano-liposome and unencapsulated free drug were separated by ultrafiltration centrifugation, and the content was determined by high performance liquid chromatography. Precisely measure 500 μL of the flexible nanoliposome suspension of finasteride into a 50KD ultrafiltration tube, centrifuge at low speed for 15 minutes, take the entrapped flexible nanolipos...
Embodiment 5
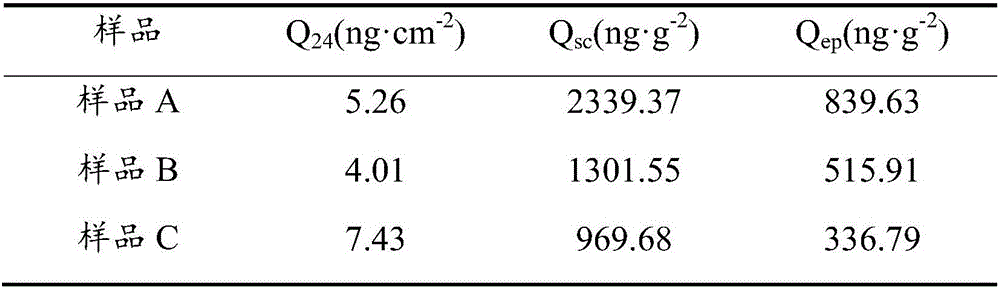
[0053] In vitro transdermal permeability study of finasteride nanoliposome gel:
[0054] The finasteride nanoliposome of embodiment 1 is made into gel, and preparation method is as follows:
[0055] The preparation of gel base: take 10 parts by weight of carbomer as the base, 50 parts by weight of glycerol as a wetting agent, 1 part by weight of sodium benzoate as a preservative, 13.5 parts by weight of triethanolamine as a pH regulator, add 17.5 parts by weight of water, Stir to get the gel matrix;
[0056] To the above gel matrix, add Example 1 to prepare finasteride nanoliposomes, add an appropriate amount of water, and stir evenly to obtain finasteride nanoliposome gel (sample A).
[0057] Prepare finasteride flexible nano-liposome gel (sample A), finasteride common liposome gel (sample B, the flexible material of the present invention is not contained in this sample formulation) and finasteride common liposome gel respectively. Gel (sample C, the sample directly disperses...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com