Production method of biodegradable nanometer fiber diaphragm applied to neurosurgery indirect vascular bypass
A nanofiber membrane and neurosurgery technology, applied in the field of nanobiomedicine, can solve the problems of lack of angiogenesis, stimulation, and inability to act directly, and achieve good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
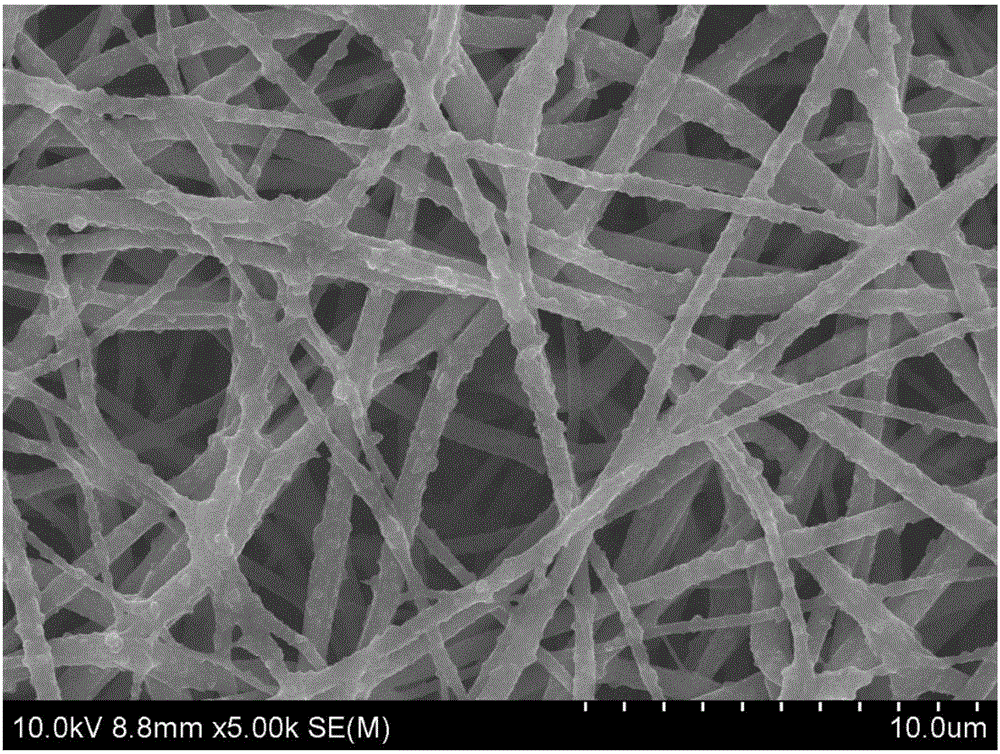
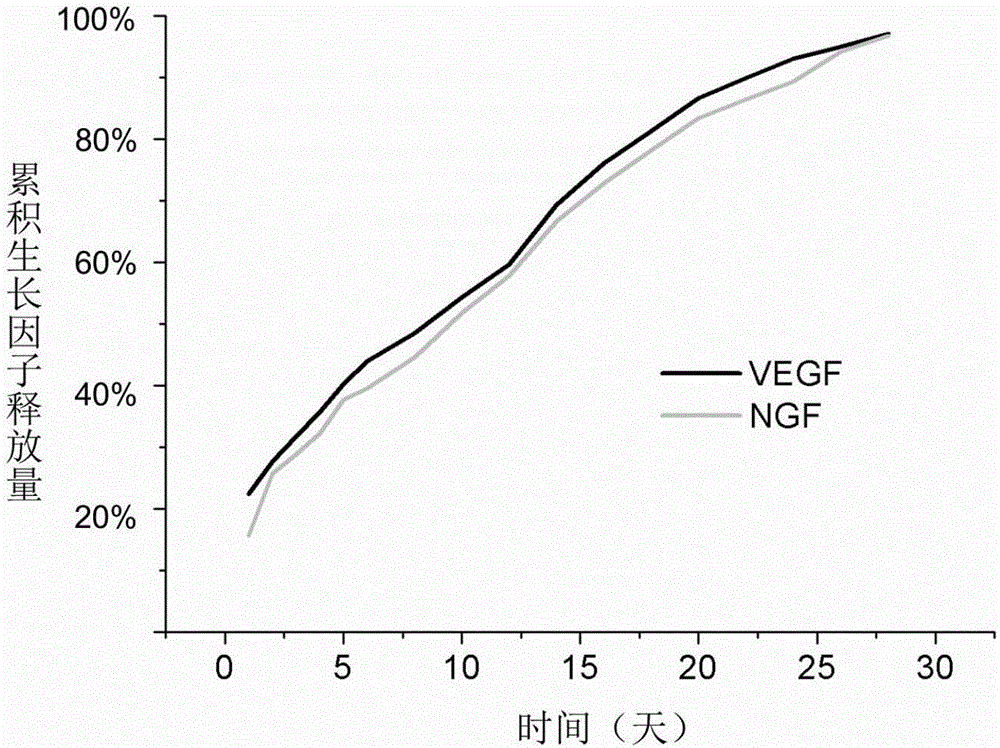
[0030] Using PCL as a raw material, it was dissolved in a mixed solution of chloroform / N,N-dimethylformamide (volume ratio 9:1) to prepare a solution with a concentration of 6% (wt). Add this mixture solution to the electrospinning syringe controlled by the syringe pump, set the voltage of the high-voltage generator to 15KV, and the collection distance to 15cm, and a membrane composed of ultrafine fibers with a diameter of nanometer scale can be obtained, and then the The membrane was washed several times with deionized water and dried in vacuum before use. The dried membrane is immersed in polyallylamine (PAH, 0.1-1 mg / ml) solution for 30-60 minutes, and then soaked in deionized water for 10 minutes. Then VEGF and NGF are made into a mixed growth factor solution with a concentration of 0.1-10 μg / ml, the membrane is soaked in the growth factor solution for 10-20 minutes for adsorption, and then washed with deionized water. Then immerse in polystyrene sodium sulfonate (PSS, 0....
Embodiment 2
[0032] PLGA is used as a raw material and dissolved in hexafluoroisopropanol to prepare a solution with a concentration of 7% (wt). Add this mixture solution to the electrospinning syringe controlled by the syringe pump, set the voltage of the high-voltage generator to 14KV, and the collection distance to 15cm, a membrane composed of ultrafine fibers with a diameter of nanoscale can be obtained, and then the The membrane was washed several times with deionized water and dried in vacuum before use. The dried membrane is immersed in polyallylamine (PAH, 0.1-1 mg / ml) solution for 30-60 minutes, and then soaked in deionized water for 10 minutes. Then VEGF and NGF are made into a mixed growth factor solution with a concentration of 0.1-10 μg / ml, the membrane is soaked in the growth factor solution for 10-20 minutes for adsorption, and then washed with deionized water. Then immerse in polystyrene sulfonate sodium (PSS, 0.1-1mg / ml) solution, growth factor solution and chitosan (Chit...
Embodiment 3
[0034] PCL and collagen (collagen) were used as raw materials (ratio: 2:1), dissolved in hexafluoroisopropanol to prepare a solution with a concentration of 7% (wt). Add this mixture solution to the electrospinning syringe controlled by the syringe pump, set the voltage of the high-voltage generator to 15KV, and the collection distance to 15cm, and a membrane composed of ultrafine fibers with a diameter of nanometer scale can be obtained, and then the The membrane was washed several times with deionized water and dried in vacuum before use. The dried membrane is immersed in polyallylamine (PAH, 0.1-1 mg / ml) solution for 30-60 minutes, and then soaked in deionized water for 10 minutes. Then VEGF and NGF are made into a mixed growth factor solution with a concentration of 0.1-10 μg / ml, the membrane is soaked in the growth factor solution for 10-20 minutes for adsorption, and then washed with deionized water. Then immerse in sodium alginate / PSS mixed solution (sodium alginate, 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com