Fluorenyl donor/receptor type nano polymer as well as preparation method and application thereof
A nano-polymer, donor-acceptor-type technology, applied in the field of donor-acceptor-type fluorenyl nano-lattice materials and its preparation, can solve the problem of low reactivity of acceptor fragments, low ring-forming yield of cyclic molecules, difficulty in separation, etc. problems, achieve the effects of reducing film solvent dependence, modularization of synthesis methods, and high scalability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation method of the fluorenyl donor-acceptor nanopolymer provided in the embodiment of the present invention includes the following steps: polymerize the nanolattice fragments (II) and (III) with reserved halogen end groups to obtain the polymer, The reaction scheme is as follows:
[0043]
[0044] Wherein, X is Cl, Br or I, and Y is borate, tin reagent, halogen or hydrogen atom.
Embodiment 1
[0046] When W is C; Ar 1 、Ar 2 For p-bromooctyloxybenzene, Ar 3 For 4,7-bis(4-octylthiophen-2-yl)benzo[c][1,2,5]thiadiazole, Ar 4 is a hydrogen atom, and the structure of the nano-lattice polymer is as follows:
[0047]
[0048] The nano-lattice polymer P1 is composed of a "口"-shaped polymeric precursor M 1 Benzodithiophene unit M with bilateral tin reagents 2 Carry out Stille coupling reaction and make, and concrete reaction route is as follows:
[0049]
[0050] Wherein, prepare " mouth " font polymerization precursor M 1 The specific reaction scheme is as follows:
[0051]
[0052]
[0053] The specific preparation method is: "口" font polymer precursor M 1 The synthesis of 3-alkylthiophene is obtained through a five-step reaction. The 3-alkylthiophene is lithiated by a low-temperature lithium reagent to obtain 1. The Stille coupling reaction connects the dibrominated benzothiadiazole unit to obtain 2. The Grignard reagent nucleophilic attack on the dibrom...
Embodiment 2
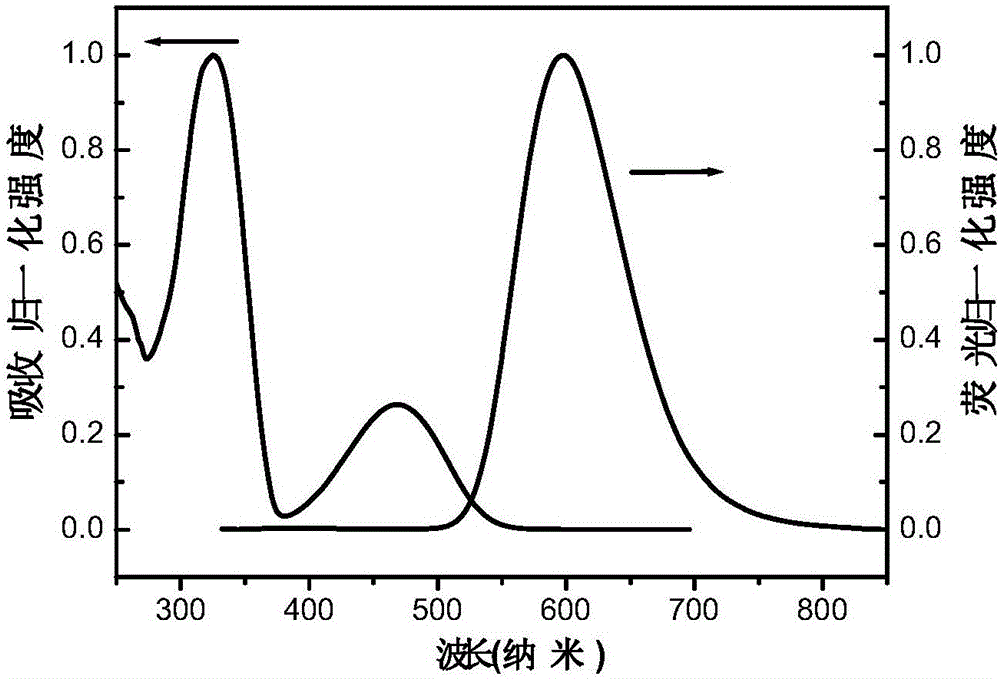
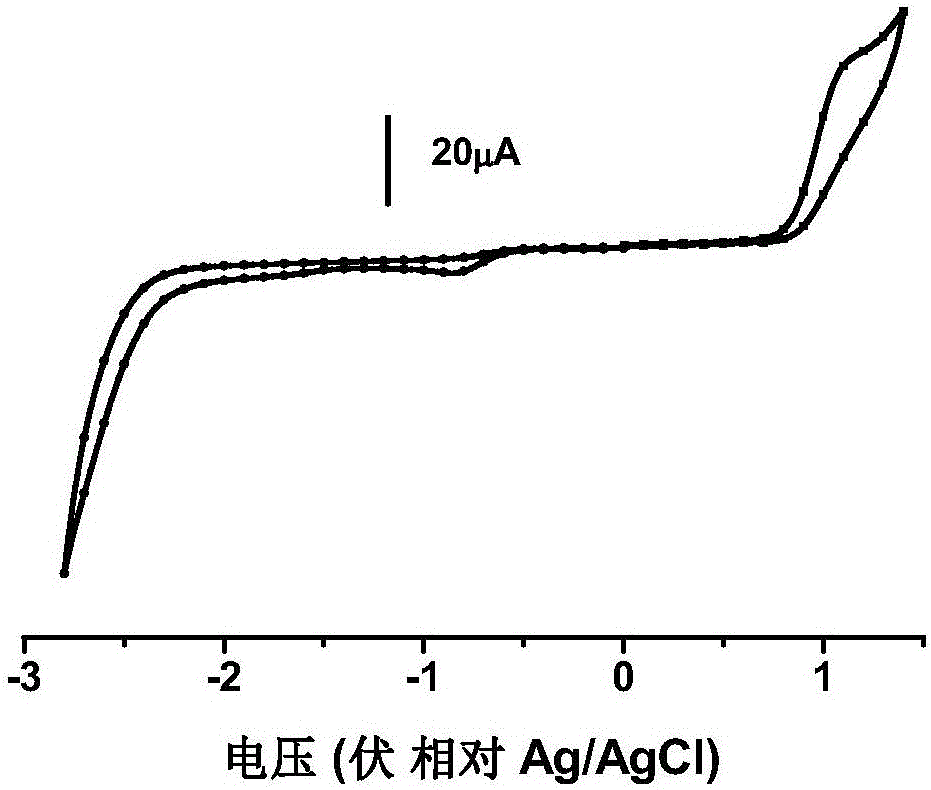
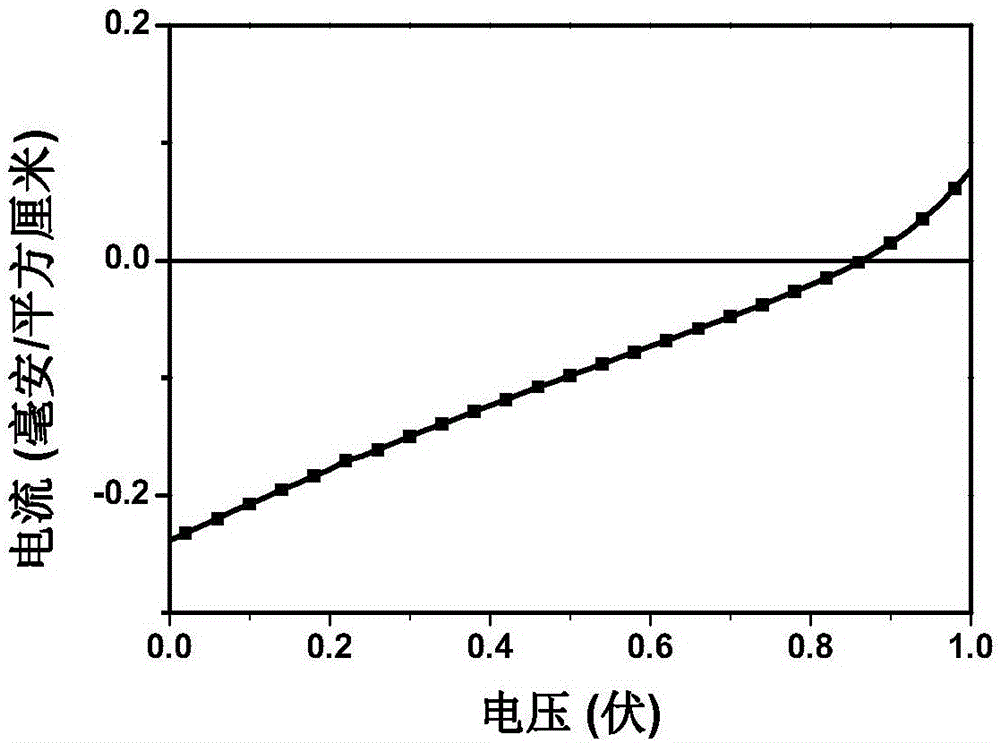
[0063] The nanolattice polymer P1 was made into an accurate 1 μM dichloromethane dilute solution, and the oxygen was removed by flushing with argon. The absorption and emission spectra were measured by Shimadzu UV-3150 ultraviolet-visible spectrometer and RF-530XPC fluorescence spectrometer. The peak value of the ultraviolet absorption spectrum of the nano-lattice polymer in dichloromethane is 325nm and 477nm, and the peak value of the fluorescence spectrum is 606nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com