High-selectivity fluorescence probe for detecting cyanide ions in ratio mode and synthesis method and application thereof
A technology of cyanide ions and fluorescent molecular probes, which is applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, and luminescent materials, can solve the problems of influence and poor selectivity, and achieve stable optical properties, high sensitivity, and synthetic products. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Synthesis of Fluorescent Molecular Probes
[0047] Dissolve N-butyl-4-methylamino-1,8-naphthalimide (0.28g, 1.0mmol) and trifluoroacetic anhydride (0.28mL, 2.0mmol) in dichloromethane (10mL), add triethyl The amine (1 mL) was reacted at room temperature for 1 h. After the reaction was over, the solvent was distilled off under reduced pressure and separated by column chromatography (dichloromethane as the eluent) to obtain the product 0.32 as a white solid (yield: 85%). The product structural formula is as follows:
[0048]
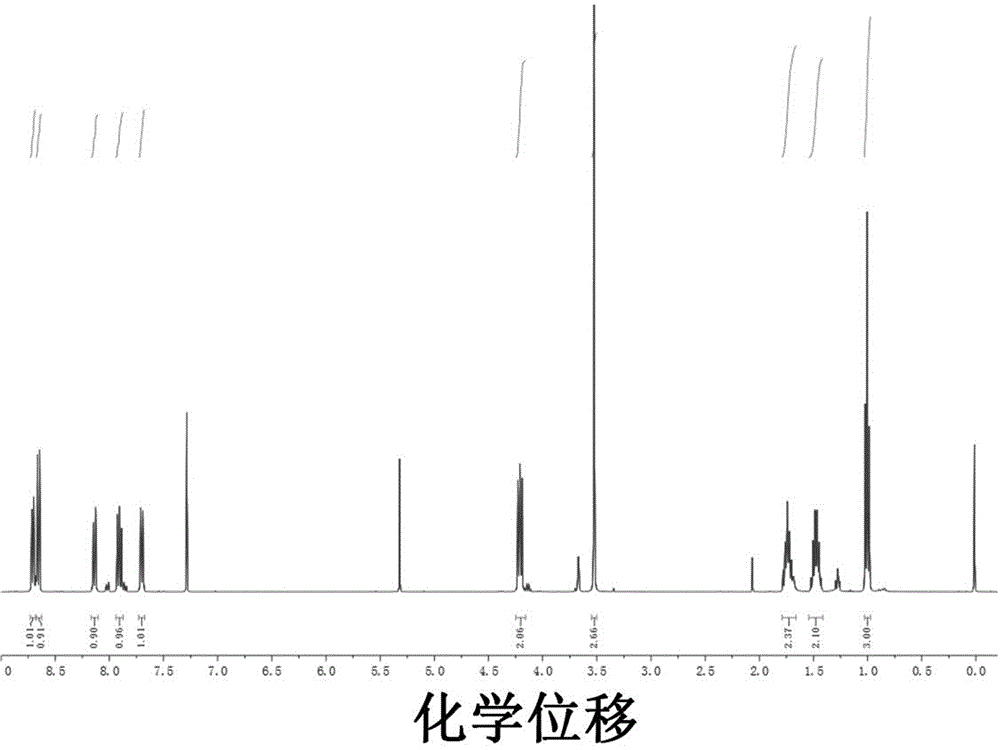
[0049] 1 H NMR (400Hz, CDCl 3 ): δ8.70(dt, J=5.7, 2.8Hz, 1H), 8.66(t, J=5.7Hz, 1H), 8.14(dd, J=8.4, 0.8Hz, 1H), 7.91(dd, J= 8.4,7.3Hz,1H),7.70(d,J=7.7Hz,1H),4.20(dd,J=16.7,9.0Hz,2H),3.52(s,3H),1.79–1.66(m,2H), 1.54–1.41(m,2H),1.00(t,J=7.4Hz,3H).HRMS[ESI]:m / z,calcd for[M-H] - 363.0957; found 363.0973.
Embodiment 2
[0050] Embodiment 2: Fluorescent detection of probes to cyanide ions
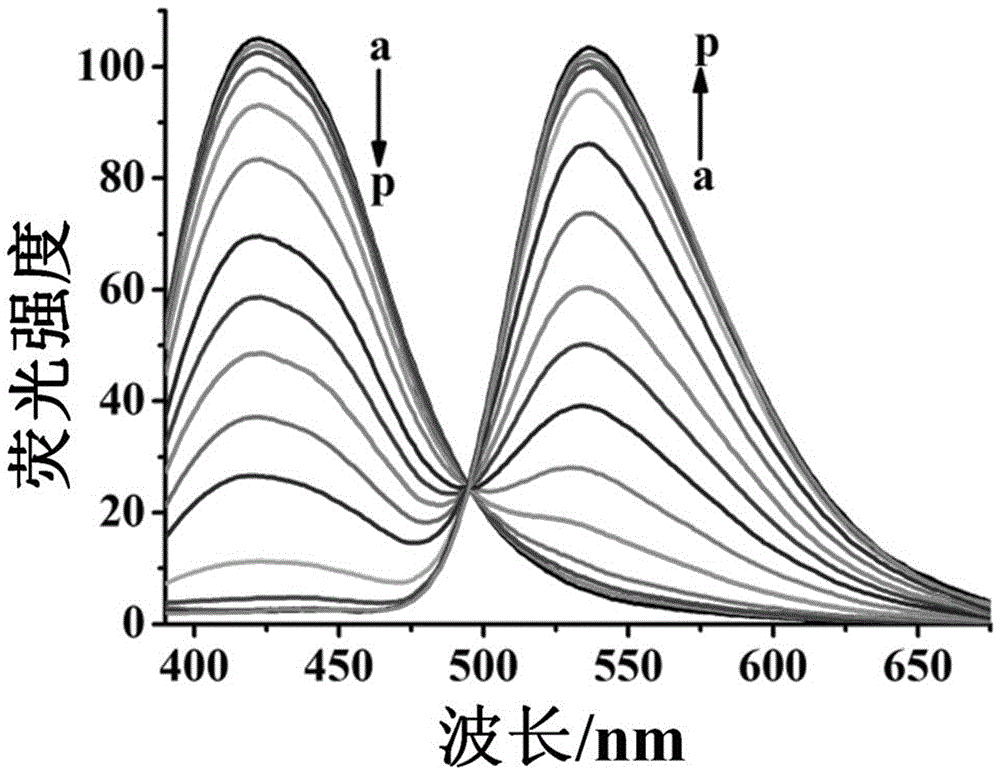
[0051] The molecular probe prepared above was dissolved in a mixed solution of water and dimethyl sulfoxide (H 2 O / DMSO=9 / 1), prepared to 5μmol L -1 probe solution. Add 2 mL of prepared 5 μmol L to a 3 mL cuvette -1 Probe solution, and then add different concentrations of cyanide ions, mix and incubate for 10min, test its fluorescence spectrum, the results are as follows image 3 shown. The ratio of the fluorescence emission intensity of the solution at 535nm and 425nm is plotted against the concentration of cyanide ions, and the concentration of cyanide ions is 1.0–80 μmol L -1 In the range, there is a good linear relationship between the two ( Figure 4 ), according to the standard diagram, the quantitative detection of cyanide ion in the sample to be tested within this concentration range is realized, and the solution changes from colorless to yellow, which is also suitable for naked eye detection. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com