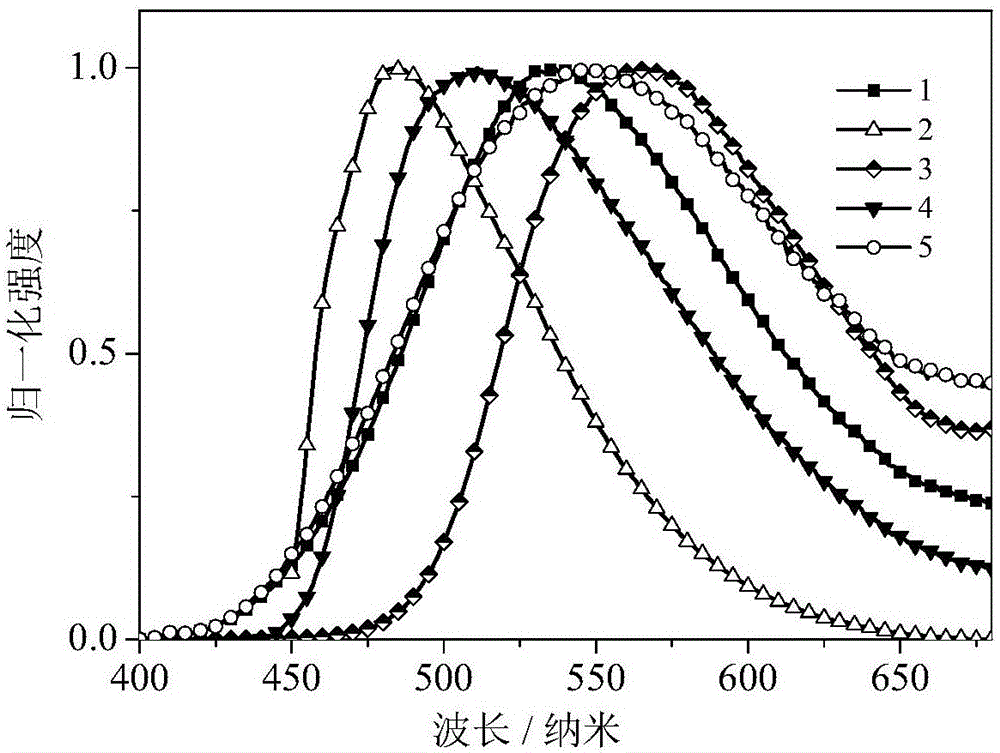
Phosphine-containing benzophenone organic light-emitting material, synthesis method and applications thereof
A technology of benzophenones and luminescent materials, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of high hole transport efficiency, achieve high fluorescence quantum efficiency, simplify the structure and preparation process, and achieve high electronic efficiency. The effect of transmission performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) Synthesis of intermediate [4-fluoro-4'-iodobenzophenone]
[0050]
[0051] Add 4-iodobenzoyl chloride (5.00g, 18.8mmol) into a 250mL dry three-necked flask, then add fluorobenzene (5.41g, 56.3mmol), stir, add anhydrous aluminum chloride (3.88g, 28.2mmol), Raise the temperature to 40-50°C and react for 5-6 hours. After the reaction, add 50ml of dichloromethane into the three-neck flask to dissolve, slowly add dilute hydrochloric acid, and stir until there is no precipitation. Then the reaction solution was poured into a separatory funnel, extracted 3 times with dichloromethane, and washed 2-3 times with dilute hydrochloric acid until the aqueous layer became colorless. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was spin-dried in a rotary evaporator to obtain 5.06 g of a yellow-white solid. Yield 83%.
[0052] (2) Synthesis of intermediate [4-iodo-4'-carbazolylbenzophenone]
[0053]
[0054] Add carbazole (1.54g, 9....
Embodiment 2
[0059]
[0060] The product of Example 1 (0.40 g, 0.75 mmol) was added into a round bottom flask, and dissolved in 20 ml of tetrahydrofuran. 6 mL of hydrogen peroxide aqueous solution (30%) was added, and after stirring for 5 hours, 50 mL of dichloromethane and 50 mL of water were added to the reaction liquid, followed by liquid separation. The dichloromethane layer was spin-dried with a rotary evaporator to obtain a white powder. The white powder was recrystallized from dichloromethane / n-hexane to obtain 0.35 g of a white solid with a yield of 85%.
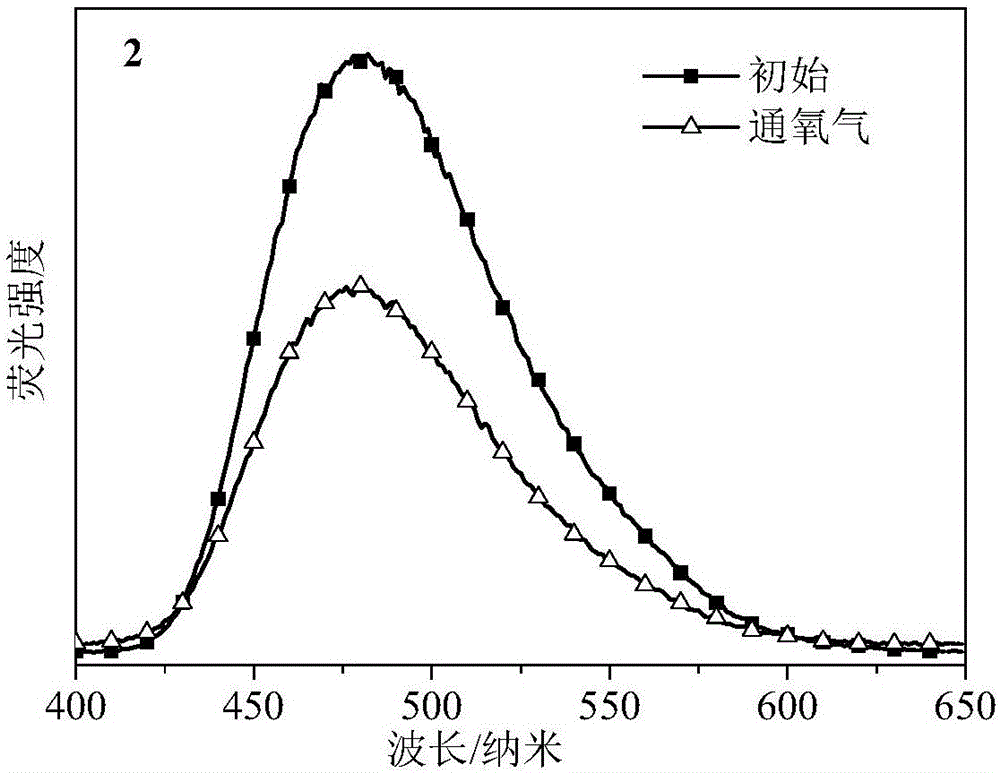
[0061] The fluorescence emission spectra of the toluene solution of the product of this embodiment before and after oxygenation are as follows: image 3 As shown, it is shown that it has a specific response to oxygen and the like.
Embodiment 3
[0063] (1) Synthesis of intermediate [4-iodo-4'-phenothiazinylbenzophenone]
[0064]
[0065] Referring to step (2) of Example 1, 4-iodo-4'-phenothiazinylbenzophenone was synthesized by using phenothiazine instead of carbazole. (49% yield)
[0066] (2) Synthesis of the target product
[0067]
[0068] With reference to step (3) of Example 1, 4-iodo-4'-phenothiazinylbenzophenone was used to replace 4-iodo-4'-carbazolylbenzophenone to synthesize 4-diphenylphosphine-4'- Phenothiazinylbenzophenone. (Yield rate is 63%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com