Method for separating and purifying vitamin K2 in bacillus subtilis natto
A technology for separation and purification of Bacillus natto is applied in the field of bioengineering to achieve the effects of high biological activity, simple process operation and convenient industrial application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
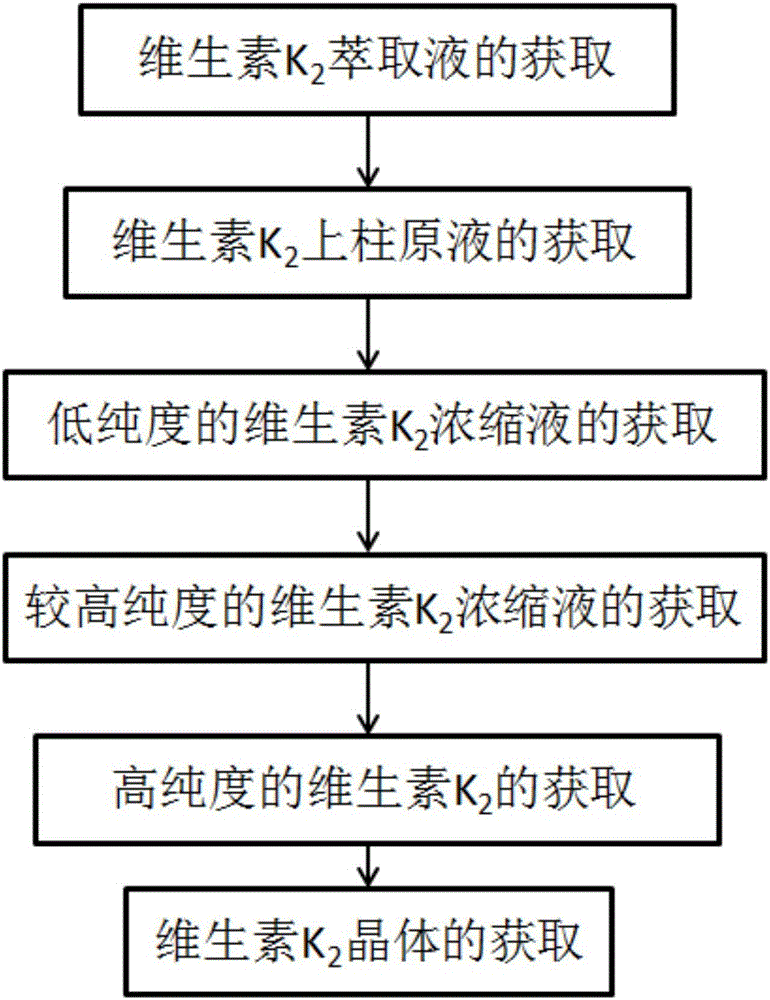
[0041] Vitamin K isolated and purified from Bacillus natto 2 method, such as figure 1 shown, including the following steps:
[0042] (1) Vitamin K 2 Extraction
[0043] Taking Bacillus natto fermentation broth as vitamin K 2 Extract the original solution, centrifuge to obtain the bacteria, after drying, use ethanol to extract to obtain vitamin K 2 Extraction solution; the extraction method is static extraction, the extraction time is 20min, and the extraction frequency is 1 time.
[0044] (2) Vitamin K 2 Obtaining the stock solution on the column
[0045] Vitamin K 2 The extract is passed through a membrane separation device (membrane pore size ≤ 0.45 μm) to remove insoluble impurities, and the obtained vitamin K 2 The filtrate was evaporated to dryness under reduced pressure, and then redissolved with methanol to obtain vitamin K 2 Stock solution on the column;
[0046] (3) Low-purity vitamin K 2 Acquisition of Concentrate
[0047] Vitamin K 2 The stock solution ...
Embodiment 2
[0057] Vitamin K isolated and purified from Bacillus natto 2 method, such as figure 1 shown, including the following steps:
[0058] (1) Vitamin K 2 Extraction
[0059] Taking Bacillus natto fermentation broth as vitamin K 2 Extract the original solution, centrifuge to obtain the bacteria, after drying, extract with toluene to obtain vitamin K 2 Extraction solution; the extraction method is static extraction, the extraction time is 60min, and the extraction frequency is 3 times.
[0060] (2) Vitamin K 2 Obtaining the stock solution on the column
[0061] Vitamin K 2 The extract is passed through a membrane separation device (membrane pore size ≤ 0.45 μm) to remove insoluble impurities, and the obtained vitamin K 2 The filtrate was evaporated to dryness under reduced pressure, then redissolved with acetone to obtain vitamin K 2 Stock solution on the column;
[0062] (3) Low-purity vitamin K 2 Acquisition of Concentrate
[0063] Vitamin K 2 The stock solution on the...
Embodiment 3
[0073] Vitamin K isolated and purified from Bacillus natto 2 method, such as figure 1 shown, including the following steps:
[0074] (1) Vitamin K 2 Extraction
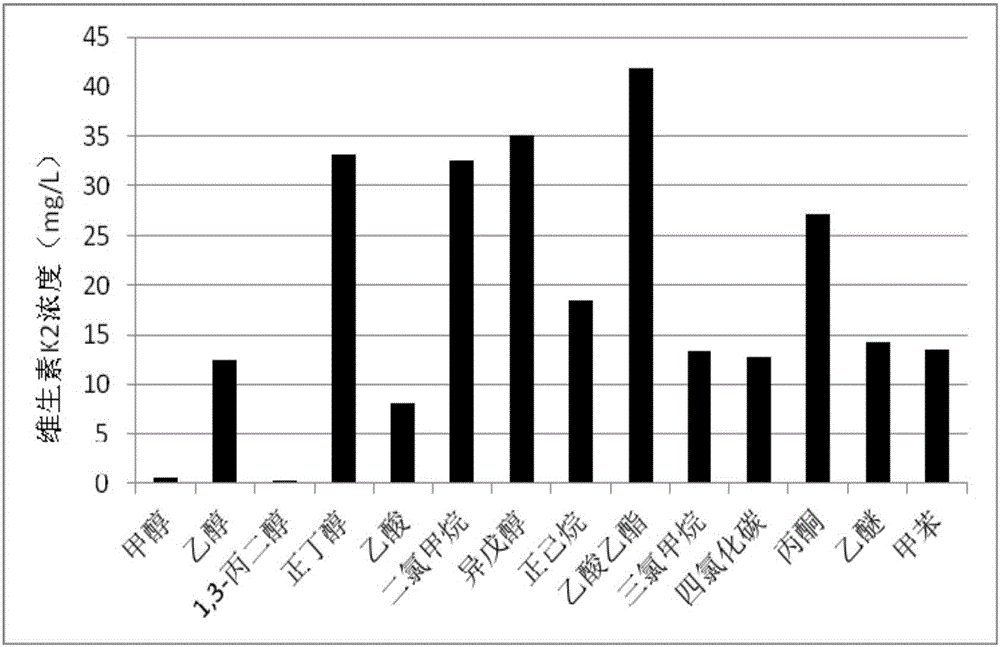
[0075] Taking Bacillus natto fermentation liquid (composition: soybean powder 65g / L, corn flour 60g / L, peptone 50g / L, fermentation period: 7-9 days) as vitamin K 2 Extract the original solution, centrifuge to obtain the bacteria, after drying, extract according to the following steps:
[0076] ① Weigh several portions of 1.0g of dry bacteria into a 50ml centrifuge tube, add 5ml of different organic solvents to stand for extraction for 50min, extract twice, and make 3 parallel samples; the different organic solvents are methanol, ethanol, 1,3 propylene glycol, n-butanol, acetic acid, dichloromethane, isoamyl alcohol, n-hexane, ethyl acetate, chloroform, carbon tetrachloride, acetone, ether, toluene;
[0077] ② Dilute the obtained extract to 5ml to obtain the test solution;
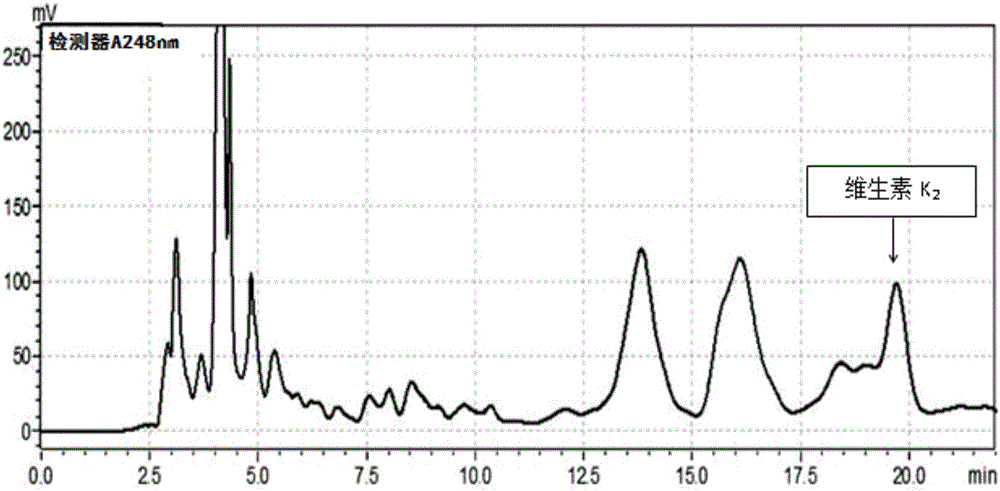
[0078] ③Use the HPLC method (mobile ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com