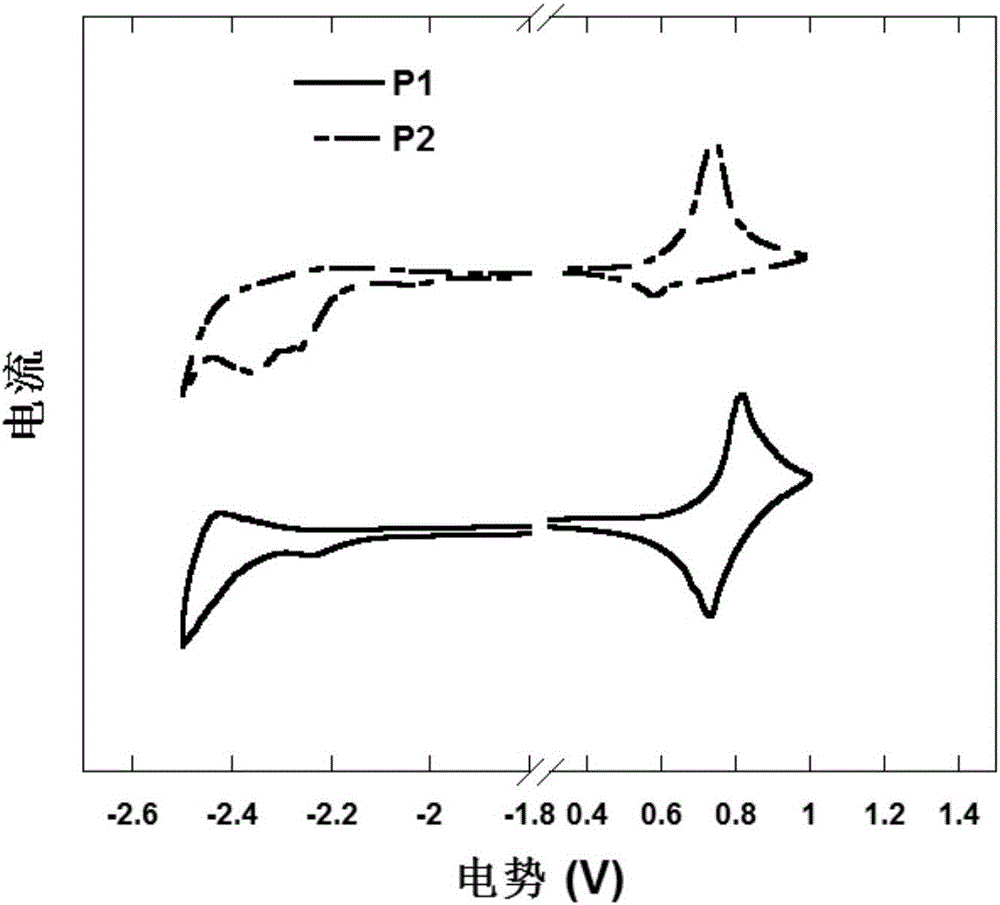
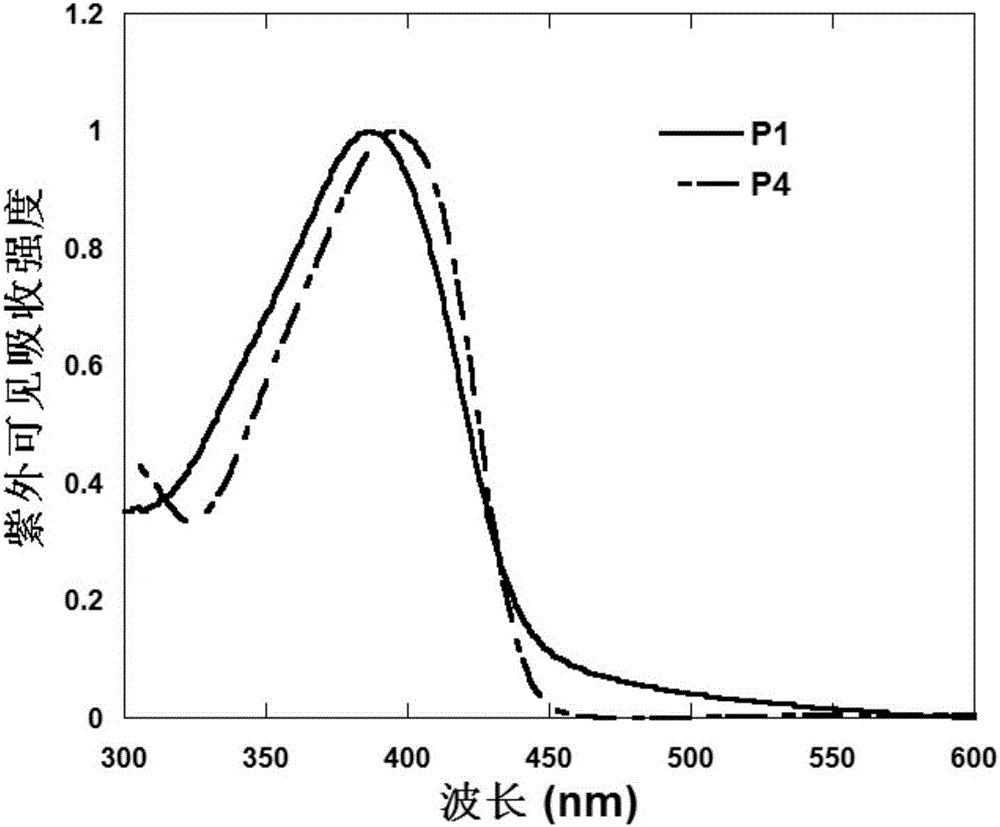
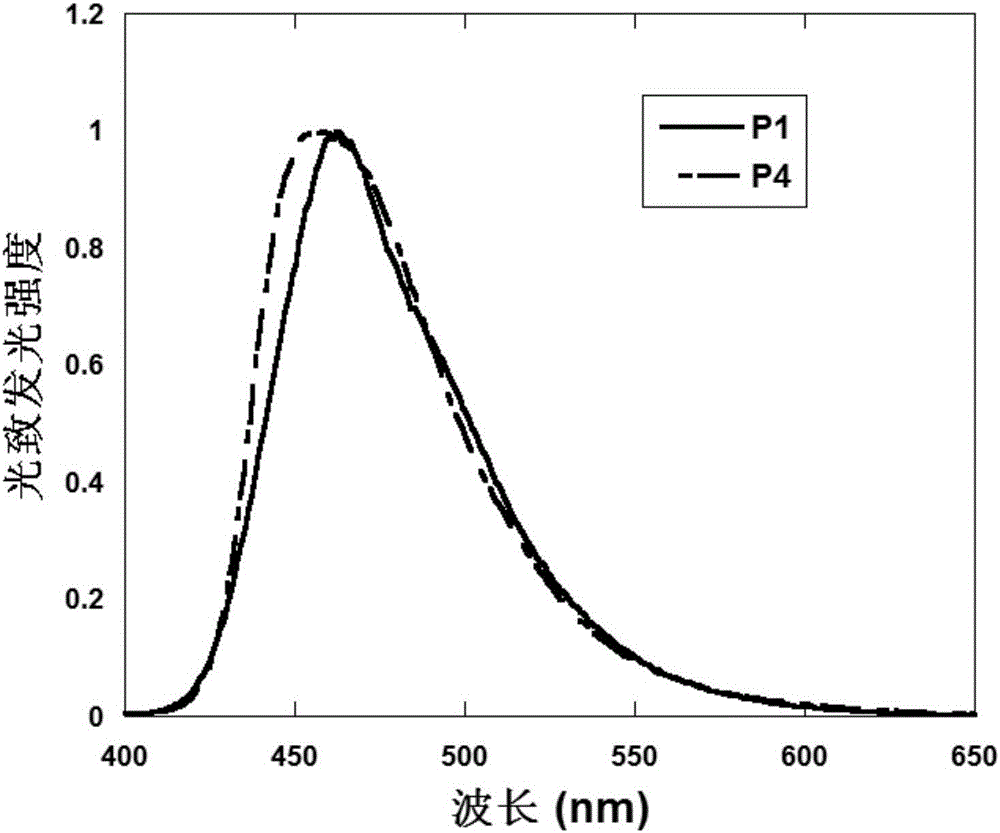
Triphenylamine polymers containing oligomeric ether side chains and application of triphenylamine polymers in preparation of organic photoelectric device by solution method
A technology of triphenylamine and oligopolyether, which is applied to triphenylamine polymers and its application field in the preparation of organic optoelectronic devices by solution method, can solve the problems of solvent erosion, large material loss and high energy consumption of the active layer, and achieves high efficiency. Effect of hole mobility, ease of synthesis, high hole transport ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of 4,4'-dibromo-4"(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)-triphenylamine (2)
[0030]
[0031] (1) Synthesis of 4,4'-dibromo-4"-hydroxytriphenylamine (1): Under nitrogen protection, 4,4'-dibromo-4"-methoxytriphenylamine (4.33g, 10mmol) Dissolved in 50ml of anhydrous dichloromethane, then slowly dropwise added in the solution of boron tribromide (5g, 20mmol) dissolved in 10ml of anhydrous dichloromethane at room temperature, when the boron tribromide solution was added dropwise , continue to react at room temperature for 8 hours; stop the reaction, pour the reaction solution into ice water, extract three times with dichloromethane, then wash three times with saturated aqueous sodium chloride solution and water, dry in anhydrous magnesium sulfate, filter, The dichloromethane solution was removed by rotary evaporation under reduced pressure, and the crude product was purified by column chromatography. The eluent was petroleum ether:dichloromethane=1:3, and a gra...
Embodiment 2
[0034] 4,4'-Di-(4,4,5,5-1,3,2-dioxaborolane-diyl)-4”-(2-(2-(2-methoxyethoxy) Synthesis of Ethoxy) Ethoxy)-Triphenylamine (3)
[0035]
[0036] Under nitrogen atmosphere, 4,4'-dibromo-4"-(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)-triphenylamine (5.65 g, 10 mmol) was dissolved In 100mL of anhydrous tetrahydrofuran (THF) solution, cool down to -78°C, slowly add 2.5mol / L n-BuLi (14mL, 35mmol) dropwise, keep stirring at this temperature for 2 hours, and quickly dissolve 2-isopropyl Base-4,4,5,5-tetramethyl-1,3,2-dioxaborane (7.44g, 40mmol) was added, naturally raised to room temperature, stirred for 24h, quenched with 5ml of distilled water, and evaporated THF was removed, the product was extracted with dichloromethane, washed with saturated sodium chloride water three times, dried with anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation. The crude product was recrystallized in a methanol / tetrahydrofuran mixed solution to obtain a white solid, the product ...
Embodiment 3
[0038] Synthesis of 4,4'-dibromo-4"-((methoxymethoxy)methoxy)triphenylamine (5)
[0039]
[0040] (1) Synthesis of (methoxymethoxy)methoxy-4-methylbenzenesulfonate (4): under nitrogen protection, (methoxymethoxy)methanol (0.92g, 10mmol), three Ethylamine (1.01g, 10mmol) was dissolved in 20ml of anhydrous dichloromethane, cooled to 0°C in an ice bath, and then 10ml of anhydrous dichloromethane solution dissolved with p-toluenesulfonyl chloride (1.90g, 10mmol) was dropped Add it into the reaction bottle, after the dropwise addition is completed, let it rise to room temperature and react for 8 hours; extract with dichloromethane, wash with saturated sodium chloride solution for 3 times, dry over anhydrous magnesium sulfate, remove the solvent by rotary evaporation, and purify the crude product by column chromatography , the eluent was petroleum ether:ethyl acetate=5:1, and a colorless liquid was obtained with a yield of 82%. (Mass Spec - APCI: 246.4).
[0041] (2) Synthesis ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical band gap | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| aggregation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com