Method for inducing transdifferentiation of fibroblast into similar testicular interstitial cells by combination of transcription factors
A technology of Leydig cells and fibroblasts, applied in chemical instruments and methods, bone/connective tissue cells, biochemical equipment and methods, etc., can solve the problems of loss of division characteristics, immune rejection, limited quantity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、11
[0097] Example 1. Construction of 11 kinds of transcription factor lentiviral expression vectors and viral packaging
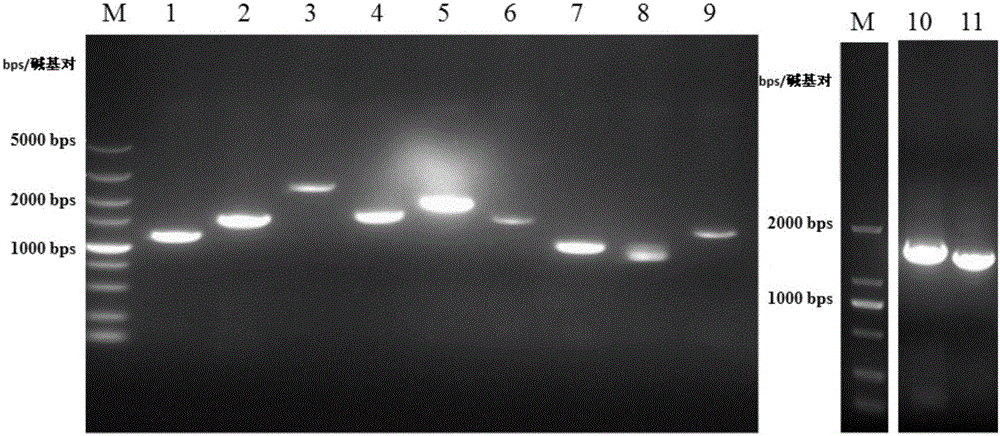
[0098] First, total RNA was obtained from mouse testes using an RNA extraction kit (Cat.#: 74134, Qiagen, purchased from Guangzhou Jianlun Biotechnology Co., Ltd., China). Then use PrimeScript TM II 1st Strand cDNASynthesis Kit (Cat.#: 6210A, Dalian Bao Biotechnology Co., Ltd., China) was used to synthesize cDNA and stored at -80°C. The upstream and downstream primers used to amplify transcription factors Dmrt1, Nr0b1, Sp1, Wt1, Nr4a1, Nr5a2, AP1, Creb1, Smad3, Nr5a1 and Gata4 genes were designed and synthesized by BGI Corporation respectively, and the forward and reverse primers Restriction sites were introduced at both ends (underlined part, Table 2).
[0099]
[0100] According to the conventional PCR method, using the mouse testis cDNA as a template, adding upstream and downstream primers (Table 2), and then setting up the PCR reaction conditions, 94...
Embodiment 2
[0102] Example 2. Separation of embryonic fibroblasts and adult fibroblasts for transdifferentiation
[0103] In order to obtain embryonic fibroblasts (mouse embryonic fibroblasts, MEFs), pregnant mice at 12.5-14.5 days of pregnancy were first sacrificed, and then the abdomen and uterus were cut open with scissors to take out the embryos. Then use sterile forceps to remove the envelope around the embryo, the limbs, head and viscera of the embryo. After washing with phosphate buffer solution, transfer the embryonic tissue pieces to a penicillin bottle and mince them with scissors, add 2 ml of trypsin, and incubate at 37°C for 10 minutes. Centrifuge at 250xg for 5 minutes, discard the supernatant, resuspend the cells with DMEM medium (Cat.#: 12100-046, invitrogen Inc., USA) containing 10% bovine serum and antibiotics, and inoculate them in a petri dish; cultivate for 24 hours After that, replace with fresh DMEM medium; when the cells are full, they can be harvested and frozen i...
Embodiment 3
[0105] Embodiment 3, construct the mouse fibroblast cell line (cyp11a1-mcherry-Fibroblasts) expressing reporter gene cyp11a1-mcherry
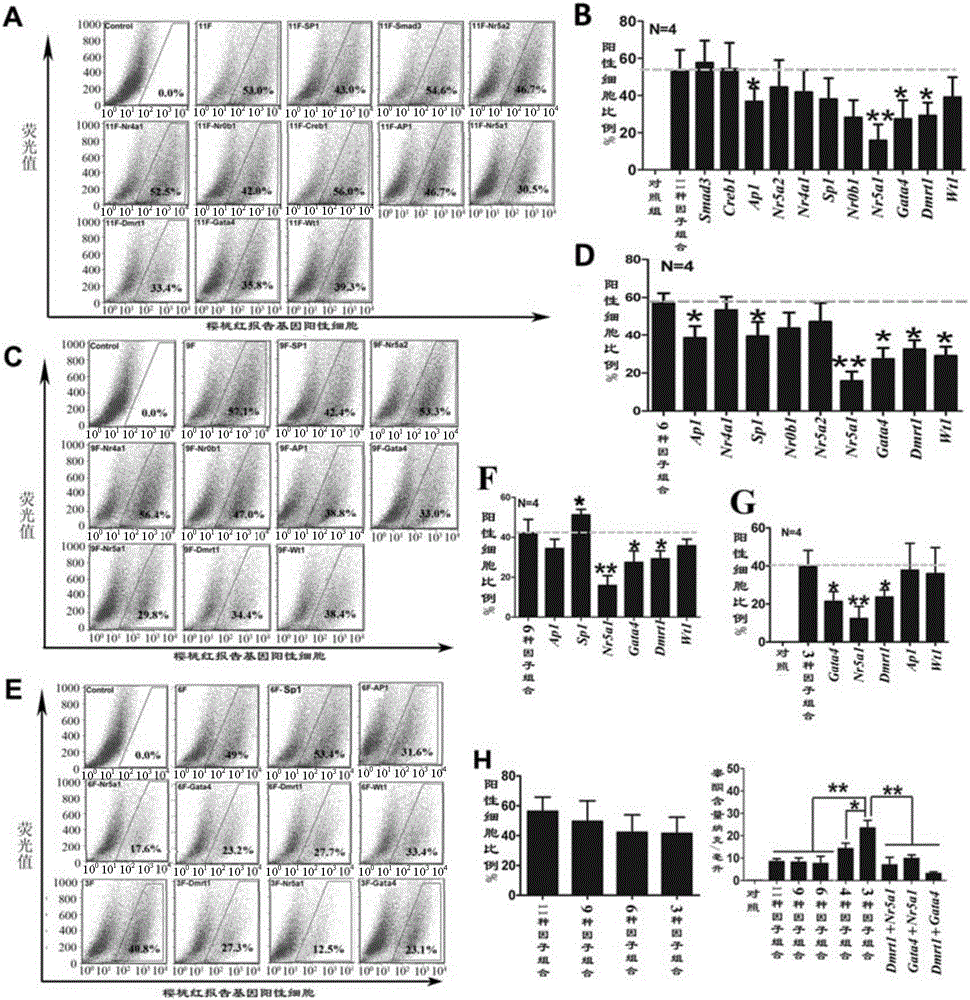
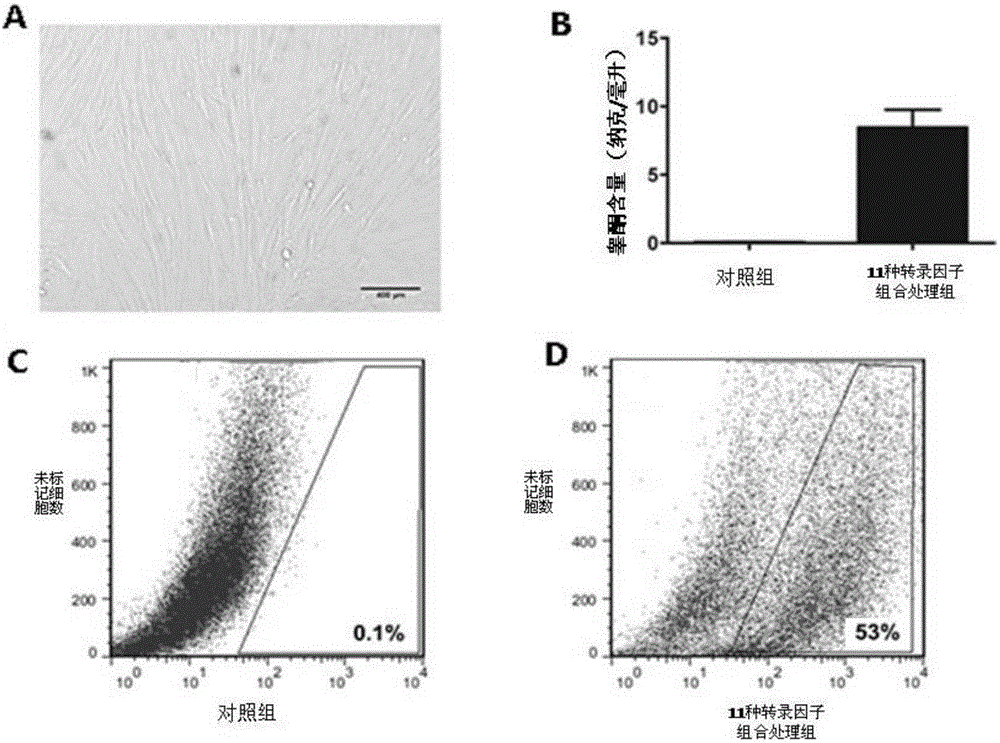
[0106] A sufficient amount of lentiviral plasmid pEZX-cyp11a1-mcherry was obtained from Example 1, and then 1×10 8 U titer of lentivirus was added to the containing 1×10 6 MEFs or TTFs (isolated in Example 2) culture dish and cultivated for 24 hours. The virus-containing DMEM medium (Cat.#: 12100-046, invitrogen Inc., USA) was discarded, replaced with fresh DMEM medium, and after continuing to cultivate for 72 hours, a final concentration of 2 micrograms / milliliter (μg / ml) was added. Puromycin was used for selection, continuous selection for 1 week ( figure 2 A). Positive clones were randomly selected for amplification, and verified by molecular biology methods such as PCR and protein hybridization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com