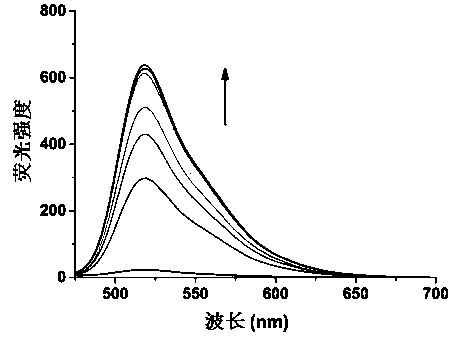
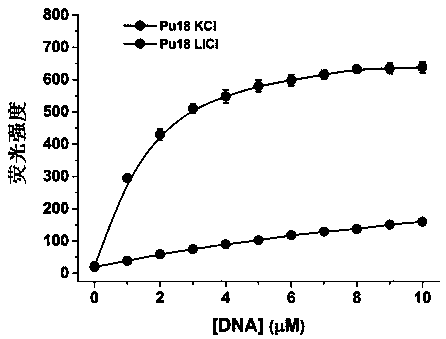
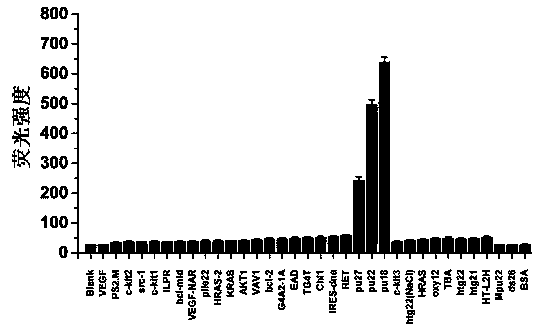
A polyaryl-substituted imidazole fluorescent probe and its preparation method and application in specific detection of g-quadruplexes
A technology of fluorescent probes and quadruplexes, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve the effects of low cost, easy storage, and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the synthesis of compound 1b
[0048] The raw material 4,4-difluorobenzil (2.5g, 10mmol) was dissolved in DMSO, 5 equivalents of N-methylpiperazine and 2 equivalents of potassium carbonate were added thereto, and reacted at 80°C for 24 hours. After the reaction, DMSO and potassium carbonate were removed by washing with water to obtain a yellow solid (2.6 g, 60%). 1 H NMR (400MHz, CDCl 3 )δ7.77(d, J=8.9Hz, 4H), 6.77(d, J=9.0Hz, 4H), 3.34(m, 8H), 2.40–2.52(m, 8H), 2.30(s, 6H). 13 C NMR (100MHz, CDCl 3 )δ193.59,154.84,132.14,123.32,113.21,54.58,46.84,46.05.ESI-MS m / z:407.2[M+H] + .
Embodiment 2
[0049] Embodiment 2: the synthesis of compound 1c
[0050] Mix 1b (0.8g, 2mmol) with 1.05 equivalents of p-propargyloxybenzaldehyde and dissolve in acetic acid, add 10 equivalents of ammonium acetate, and reflux at 115°C for 20 hours. Sodium hydroxide solution adjusted Ph to weakly alkaline, extracted with ethyl acetate, mixed with silica gel, and passed through the column with dichloromethane:methanol=20:1 eluent to obtain a light yellow solid (0.7g, 65%) . 1 H NMR (400MHz, CDCl 3 )δ7.78(d, J=8.7Hz, 2H), 7.41(d, J=8.6Hz, 4H), 6.95(d, J=8.8Hz, 2H), 6.83(d, J=8.7Hz, 4H) ,4.68(s,2H),3.41(s,1H),3.35–3.04(m,8H),2.73–2.47(m,8H),2.33(s,6H). 13 CNMR (101MHz, CDCl 3 )δ157.74, 150.05, 145.27, 128.59, 126.73, 124.03, 115.68, 115.13, 78.37, 75.77, 55.88, 55.06, 48.72, 46.10. ESI-MSm / z: 547.3[M+H] + .
Embodiment 3
[0051] Embodiment 3: the synthesis of compound 2b
[0052] Fluorescein 2a (3.3 g, 10 mmol) was dispersed in 100 mL of ethanol, 2 mL of concentrated sulfuric acid was added dropwise thereto, and refluxed for 20 hours. Then part of the ethanol was removed by rotary evaporation, and water was added to adjust Ph=5, that is, a large amount of yellow solids were precipitated. The target product (3.0 g, 83%) was obtained by suction filtration. 1 H NMR (400MHz, DMSO) δ8.18 (dd, J = 7.8, 1.1Hz, 1H), 7.86 (td, J = 7.5, 1.3Hz, 1H), 7.77 (td, J = 7.7, 1.3Hz, 1H) ,7.49(dd,J=7.5,0.9Hz,1H),6.81(s,1H),6.79(s,1H),6.73–6.37(m,4H),3.96(q,J=7.1Hz,2H), 0.86(t,J=7.1Hz,3H). 13 C NMR(101MHz,DMSO)δ165.48,150.82,134.07,133.43,131.10,131.04,130.50,130.38,103.77,61.32,13.77.ESI-MS m / z:361.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com