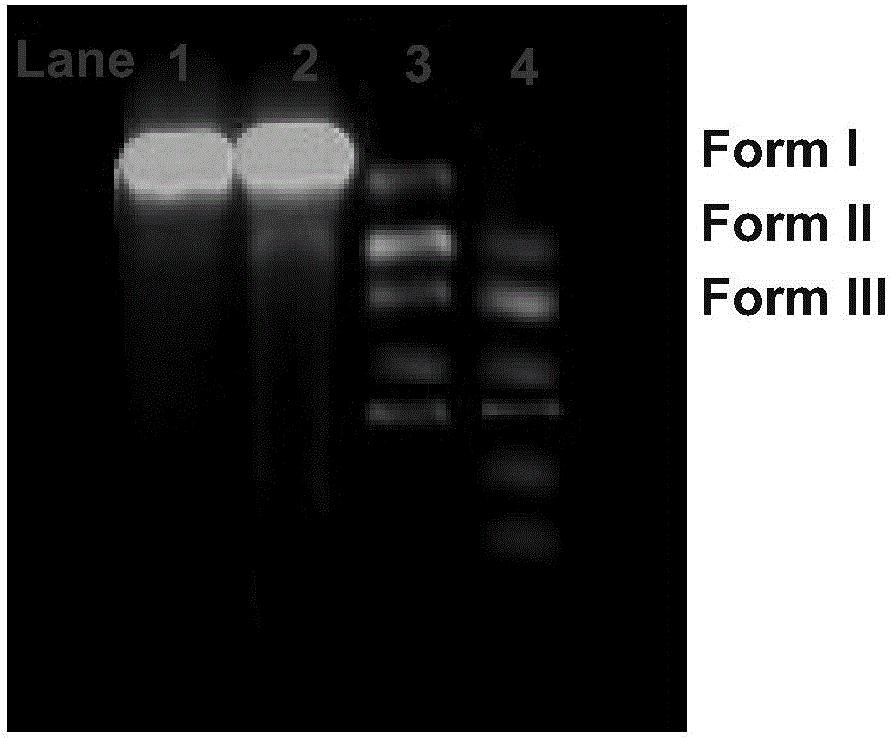
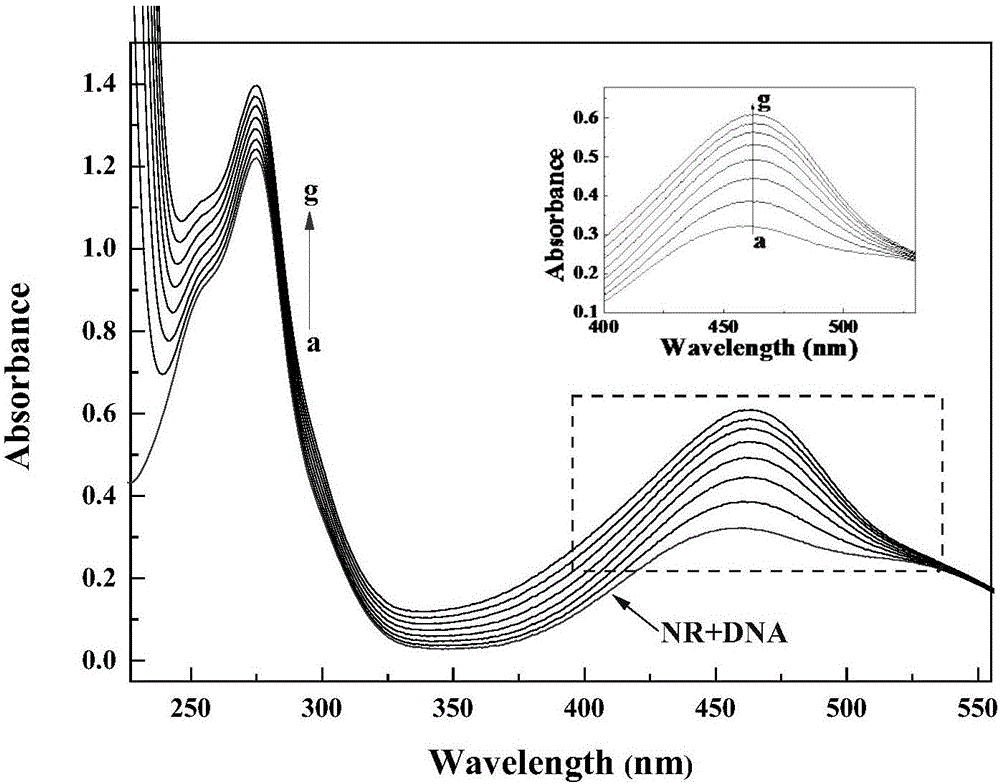
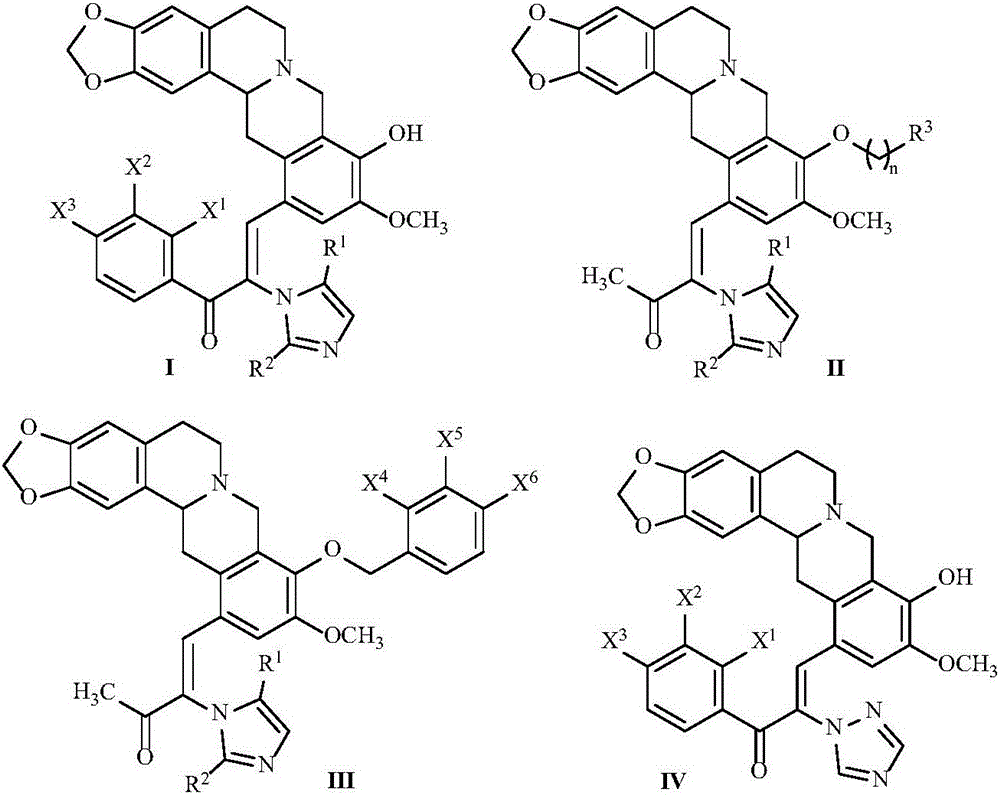
Ketene-bridged berberine azole derivatives and preparation method and application thereof
A technology of azole derivatives and enone bridging, which is applied in the chemical field, can solve the problems of poor tolerance and curative effect, many times of medication for patients, and low bioavailability, so as to achieve easy availability of raw materials, solve drug resistance, The effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1, the preparation of intermediate X
[0055]
[0056] In a 100mL round bottom flask, dissolve compound IX (2.0g, 6.1mmol) and hexamethylenetetramine (1.0g, 7.4mmol) in an appropriate amount of trifluoroacetic acid, stir at reflux for 5 hours, and then add 10% sulfuric acid solution 40mL, keep stirring at 90°C, track the reaction by thin-layer chromatography until the end of the reaction, cool to room temperature, neutralize with sodium bicarbonate, add ethyl acetate for extraction, then concentrate and dry to obtain 1.77g of intermediate X, the yield is 82.1% .
[0057] Intermediate X: brown powder; melting point 242–244°C; 1 H NMR (600MHz, DMSO-d 6 )δ: 10.12(s,1H,CHO),9.93(s,1H,OH),7.33(s,1H,berberrubine-11-H),6.99(s,1H,berberrubine-1-H),6.68(s ,1H,berberrubine-4-H),5.96(d,J=8.7Hz,2H,OCH 2 O), 4.06–4.01 (m, 1H, berberrubine-8-H), 3.96 (dd, J=16.8, 3.1Hz, 1H, berberrubine-13-H), 3.86 (s, 3H, OCH 3 ), 3.39 (d, J = 11.1Hz, 1H, chiral-H), 3.30 (d, J = 1...
Embodiment 2
[0058] Embodiment 2, the preparation of azole compounds XV and XVI
[0059]
[0060] Reference "Yin B T, Yan C Y, Peng X M, Zhang S L, Rasheed S, Geng R X, Zhou CHe. Synthesis and biological evaluation of α-triazolyl chalcones as a new type of potential antimicrobial agents and their interaction with calf thymus DNA and human serum albumin. Eur.J.Med.Chem., 2014,71,148-159 "method described in preparation. Obtain 0.69g azole compound XV (X 1 with X 3 Both are F, X 2 for H, R 6 for CH 3 ), yield 23.0%. Obtain 0.69g azole compound XVI (X 1 with X 3 Both are F, X 2 for H, R 6 for CH 3 ), yield 23.0%.
[0061] Azole compound XV: yellow powder; melting point 101–102°C; 1 H NMR (600MHz, DMSO-d 6 )δ: 8.05 (dd, J = 15.3, 8.5Hz, 1H, Ph-3-H), 7.54 (dd, J = 15.1, 5.6Hz, 1H, Ph-5-H), 7.34–7.30 (m, 1H , Ph-6-H), 6.33 (d, J=2.4Hz, 2H, CH 2 ),2.50(s,3H,CH 3 )ppm.
[0062] Azole compound XVI: yellow powder; melting point 76–77°C; 1 H NMR (600MHz, DMSO-d 6 )δ: 8.07(dd, J=...
Embodiment 3
[0063] Embodiment 3, preparation of berberine azole derivatives I-1 of enone bridge
[0064]
[0065] In a 100mL round bottom flask, add intermediate X (1.9g, 5.4mmol), the azole compound (X 1 with X 3 Both are F, X 2 , R 1 with R 2 Both are H) (1g, 4.5mmol), glacial acetic acid (1.0mL) and an appropriate amount of 1,4-dioxane, the temperature is controlled at 100°C and the reaction is stirred, followed by TLC until the end of the reaction, cooled to room temperature, and then concentrated , extraction, separation by column chromatography, and drying to obtain 0.69 g of compound I-1, with a yield of 23.3%.
[0066] Compound I-1: yellow powder; melting point 220–221°C; 1 H NMR (600MHz, DMSO-d 6 )δ: 9.64(s,1H,OH),7.87(dd,J=14.9,8.2Hz,1H,Ph-3-H),7.73(s,1H,enone-H),7.63(s,1H,imidazole -2-H), 7.38(td, J=9.9, 2.2Hz, 1H, Ph-5-H), 7.23–7.20(m, 2H, Ph-6-H, imidazole-5-H), 7.11(s ,1H,imidazole-4-H),6.87(s,1H,berberrubine-1-H),6.68(s,1H,berberrubine-4-H),6.01(s,1H,OCHO),5.97(s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com