A catalytic system for ring-opening polymerization of lactones with controllable activity
A ring-opening polymerization and catalytic system technology, applied in the field of active controllable polymerization catalytic system, can solve the problems of low polymer molecular weight, large amount of catalyst, wide molecular weight distribution, etc., and achieve wide substrate applicability, narrow molecular weight distribution, The effect of using less
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A kind of specific synthetic method of Lewis base described in embodiment 1 present invention
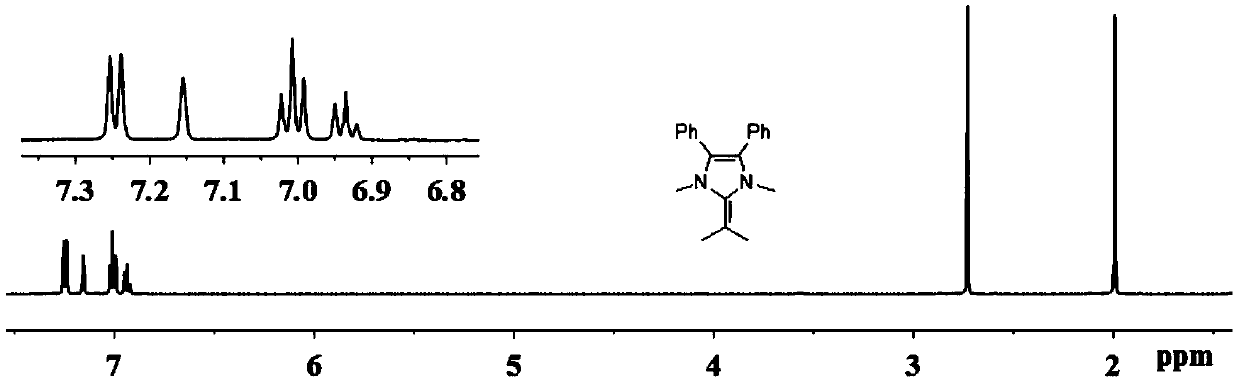
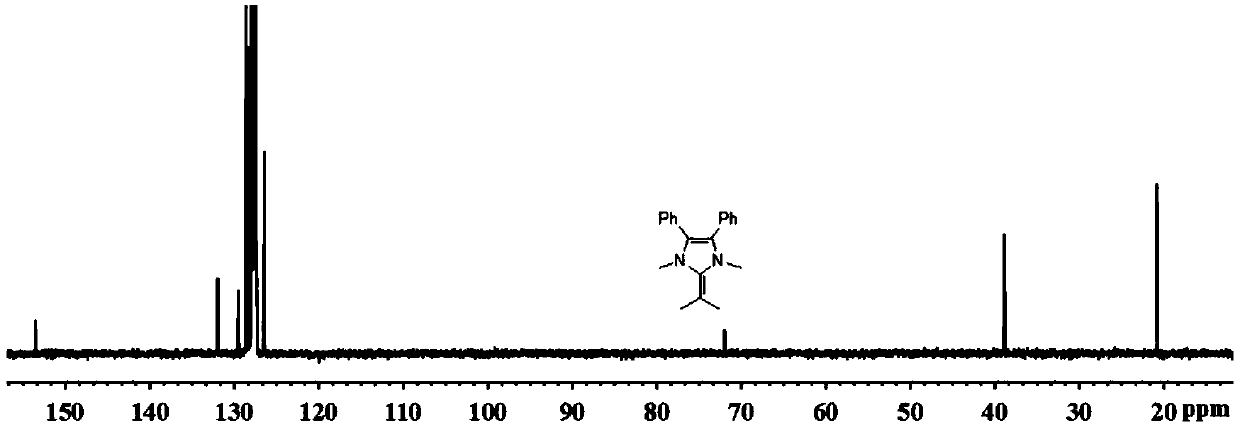
[0053] Synthesis of 1,3-Dimethyl-4,5-diphenyl-2-(2-propenyl)-2,3-dihydro-1H-imidazole.
[0054] 1) Synthesis of 2-isopropyl-4,5-diphenyl-1H-imidazole
[0055]
[0056] Take ammonium acetate (7.7g, 99.9mmol) in a 250mL pressure-resistant reaction flask, add methanol (150mL) and cool to 0°C, add isobutyraldehyde (3.6g, 49.9mmol) and dibenzoyl (10.5g, 49.9mmol) . Seal the reaction bottle, put it into an oil bath at 120°C and stir for 3h. Cool, concentrate methanol in vacuo, filter, wash with ether (3 x 30 mL), collect an off-white solid and dry in vacuo (8.0 g, 61.0%). 1 H NMR (300MHz, DMSO-d 6 )δ11.92(s,1H,NH),7.48–7.16(m,10H,Ph),3.01(sept,J=7.1Hz,1H,CHMe 2 ), 1.31 (d, J=7.1Hz, 6H, CHMe 2 ).
[0057] 2) Synthesis of 1,3-dimethyl-2-isopropyl-4,5-diphenylimidazolium iodide salt
[0058]
[0059] Take 2-isopropyl-4,5-diphenylimidazole (8.0g, 30.5mmol) in a 250mL roun...
Embodiment 2
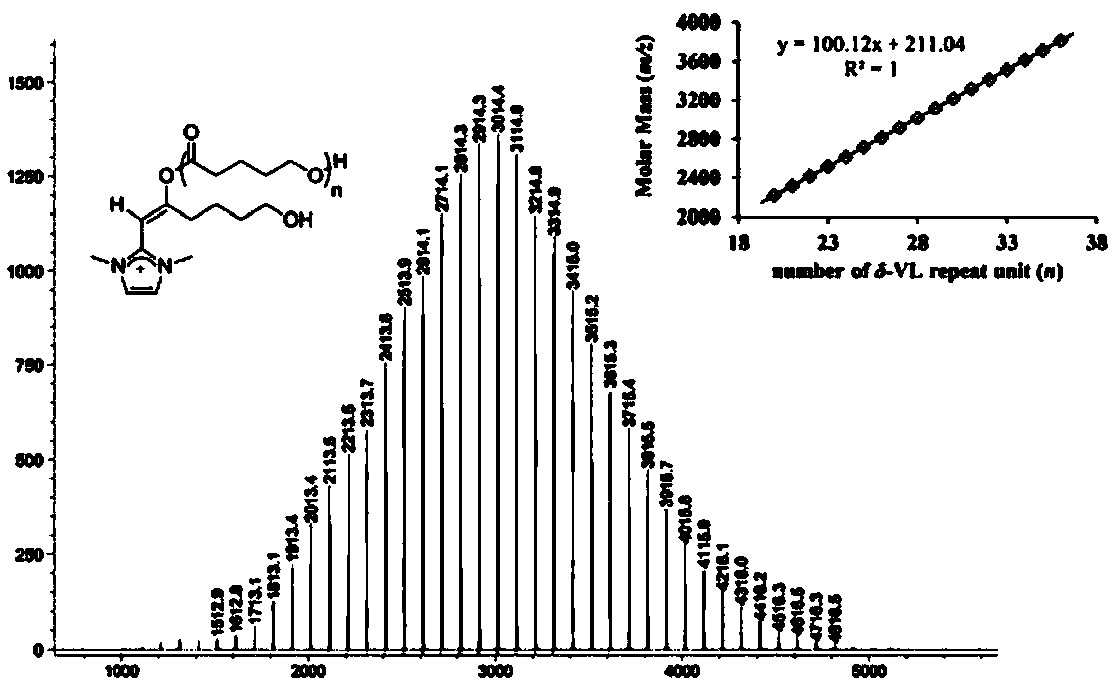
[0063] The ring-opening polymerization of embodiment 2 δ-valerolactone (δ-VL)
[0064] There are three feeding methods in the polymerization process: 1. Pre-mix Lewis acid and Lewis base for 10 minutes, and then add monomer; 2. Pre-mix Lewis acid and monomer, and then add Lewis base; 3. Pre-mix Lewis base and monomer, and then Add Lewis acid. Regardless of the feeding method, the ring-opening polymerization of lactone can be well realized.
[0065] The polymerization reaction was carried out in a glove box, and δ-valerolactone (4.0g, 40mmol) was weighed, a suitable amount of solvent was placed in a 30ml reaction bottle (the total volume of the solution was 10mL), Lewis base and Lewis acid were added respectively, and the timing was started , and stirred for a period of time until the monomer was completely converted, the reaction bottle was taken out of the glove box, and 5% HCl / methanol solution was added to terminate the polymerization reaction. The polymer was filtered of...
Embodiment 3
[0072] The ring-opening polymerization of embodiment 3ε-caprolactone (ε-CL)
[0073] The polymerization reaction was carried out in a glove box, and an appropriate amount of solvent was weighed in a 30 milliliter reaction bottle (to keep the total volume of the solution at 10 mL), adding Lewis base and Lewis acid, stirring for 10 minutes, and then adding ε-caprolactone (4.56 g, 40mmol), and start timing, stirring for a period of time until the monomer is completely converted, the reaction bottle is taken out from the glove box, and 5% HCl / methanol solution is added to terminate the polymerization reaction. The polymer was filtered off, washed thoroughly with methanol, and dried under vacuum at 60°C to constant weight. The resulting polymer passed 1 H / 13 CNMR identification, molecular weight and molecular weight distribution of the polymer were measured by gel permeation chromatography and laser light scattering. 1 HNMR (500MHz, Chloroform-d) δ4.06 (t, J = 6.7Hz, 2H), 2.30 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com