Affinity chromatography preparation method of high-activity trypsin
A trypsin, high-activity technology, applied in biochemical equipment and methods, enzymes, peptidases, etc., can solve the problems of weak purification ability, inconvenient large-scale production, low titer of finished products, etc., to avoid the zymogen precipitation step, The effect of improving market competitiveness and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
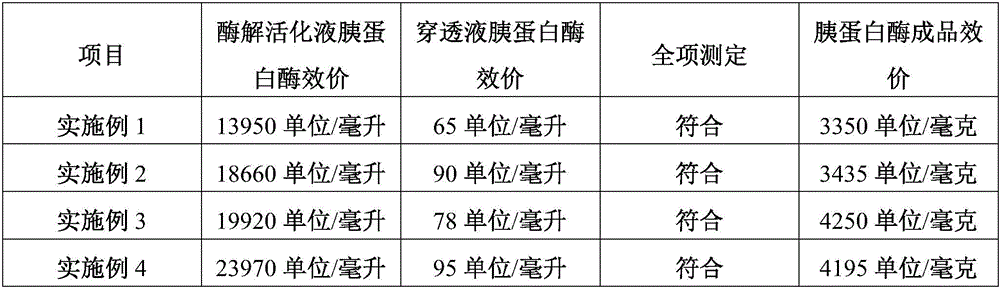
Embodiment 1
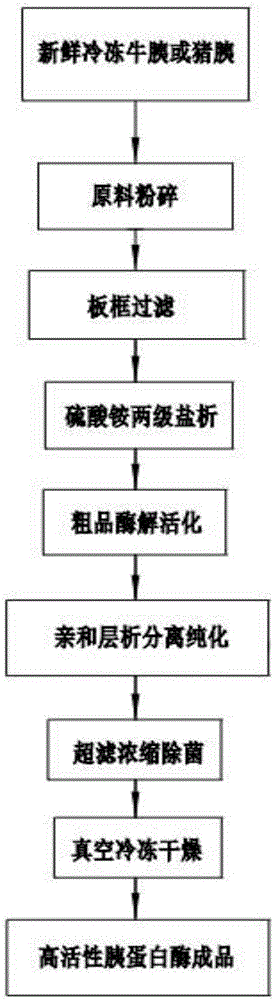
[0032] (1) Grinding of raw materials, rough extraction of trypsin: take 150 kg of fresh frozen bovine pancreas, and directly crush the frozen raw materials with a pulverizer; add 450 L of pre-cooled 0.125M sulfuric acid solution, transfer it to a 1-ton stirring container, and place it in a 4-degree cold storage Stir and extract in medium for 6 hours, place overnight; filter with plate and frame filter equipment the next day, pack the separated residue into bags and treat according to environmental protection requirements, and the obtained supernatant is the crude extract of trypsin.
[0033] (2) Two-stage salting-out of ammonium sulfate: add reagent-grade ammonium sulfate to the above-mentioned trypsin crude extract to 25% saturation, and add a small amount of diatomaceous earth to aid in filtration, place it in a 4-degree cold storage overnight and filter, and take the supernatant Then add reagent-grade ammonium sulfate to the supernatant to 65% saturation, place it overnight ...
Embodiment 2
[0039](1) Raw material crushing, rough extraction of trypsin: take 450 kg of fresh frozen bovine pancreas, and use a pulverizer to directly crush the frozen raw material into a slurry; add 1350 L of pre-cooled 0.125M sulfuric acid solution, transfer it to a 2-ton stirring container, and Stir and extract in a cold storage for 6 hours, and place overnight; the next day, filter it with a plate-and-frame filter, bag the separated residue and treat it according to environmental protection requirements, and the obtained clear liquid is the trypsin crude extract.
[0040] (2) Two-stage salting-out of ammonium sulfate: Stir and add reagent-grade ammonium sulfate to the above-mentioned trypsin crude extract to 25% saturation, and add a small amount of diatomaceous earth to aid in filtering, place it in a 4-degree cold storage overnight, and then filter. Supernatant: Add reagent-grade ammonium sulfate to the supernatant to 65% saturation, place it overnight in a 4-degree cold storage, ab...
Embodiment 3
[0046] (1) Crushing of raw materials, rough extraction of trypsin: 200 kg of fresh frozen porcine pancreas was taken, and the frozen raw materials were directly pulverized into a slurry with a pulverizer. Add 600L of pre-cooled 0.125M sulfuric acid solution, transfer it to a 1-ton mixing container, stir and extract in a 4-degree cold storage for 6 hours, and place it overnight; filter it with a plate-and-frame filter the next day, and bag the separated residue according to environmental protection requirements After treatment, the obtained supernatant is the trypsin crude extract.
[0047] (2) Two-stage salting-out of ammonium sulfate: Stir and add reagent-grade ammonium sulfate to the above-mentioned trypsin crude extract to 25% saturation, and add a small amount of diatomaceous earth to aid in filtering, place it in a 4-degree cold storage overnight, and then filter. Supernatant: Add reagent-grade ammonium sulfate to the supernatant to 65% saturation, place it overnight in a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com