A method for co-producing benzoic acid, p-toluic acid and m-toluic acid
A technology of p-toluic acid and toluic acid is applied in chemical instruments and methods, separation/purification of carboxylic acid compounds, preparation of carboxylate salts, etc. Large, high production costs, to achieve the effects of low prices, diversified product structure, and less investment in equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
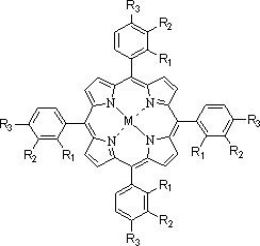
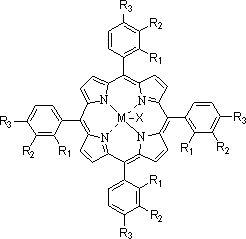
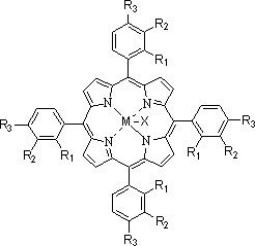
[0071] The fresh xylene added to the system is 91.4g, and the mass composition of xylene is ethylbenzene: p-xylene: m-xylene=45:50:5, and the dissolved catalyst is N -Hydroxyphthalimide, metallophthalocyanine (R) with general formula (IV) structure 1 =OH,R 2 =H, M=Ru), metalloporphyrins with general formula (III) structure (R 1 =R 3 = H, R 2 =OH,M 1 = M 2 =Mn) and cobalt naphthenate, the total concentration is 10000ppm. After the system circulation was stabilized, the mass of the oxidation reaction solution added in circulation was 488.6 g. The pressurized pure oxygen is continuously passed into the oxidation reaction kettle, the reaction temperature of the system is maintained at 130°C, the reaction pressure is 0.2MPa, and the reaction time is 3.2 hours. The implementation effect is that the conversion rate of xylene is more than 99.9%, the selectivity of each target product is more than 99%, the yield of benzoic acid is 97.8%, the yield of m-toluic acid is 90.2%, p-to...
Embodiment 2
[0073] The fresh xylene added to the system is 66.9g, and the mass composition of xylene is ethylbenzene:p-xylene:m-xylene=25:25:50, and the dissolved catalyst is Ni(Ac) 2 , N,N',N'' - trihydroxyisocyanuric acid, metallophthalocyanine (R) with general formula (IV) structure 1 = H, R 2 =Cl, M=Zn) and metalloporphyrins (R 1 =R 2 =CH 3 CH 2 , R 3 =H, M=Fe, X=Br), the total concentration is 420ppm. After the system circulation was stabilized, the mass of the oxidation reaction liquid added in circulation was 513.1 g. The pressurized pure oxygen is continuously fed into the oxidation reaction kettle, the reaction temperature of the system is maintained at 125°C, the reaction pressure is 0.6MPa, and the reaction time is 1.5 hours. The implementation effect is that the conversion rate of xylene is more than 99.9%, the selectivity of each target product is more than 99%, the yield of benzoic acid is 98.1%, the yield of m-toluic acid is 93.4%, p-toluic acid The yield was 95.1...
Embodiment 3
[0075] The fresh xylene added to the system is 169.2g, and the mass composition of xylene is ethylbenzene:p-xylene:m-xylene=20:30:50, and the dissolved catalyst is Mn(Ac) 2· 4H 2 O, metal phthalocyanine (R) with general formula (IV) structure 1 =NO 2 , R 2 =H, M=Co), metalloporphyrins with the structure of general formula (I) (R 1 =R 3 = H, R 2 =CH 3 CH 2 , M=Cu) mixture, the total concentration is 120ppm. After the system circulation was stabilized, the mass of the oxidation reaction liquid added in circulation was 410.8 g. The pressurized pure oxygen is continuously passed into the oxidation reaction kettle, the reaction temperature of the system is maintained at 175°C, the reaction pressure is 0.7MPa, and the reaction time is 1.5 hours. The implementation effect is that the conversion rate of xylene is more than 99.9%, the selectivity of each target product is more than 99%, the yield of benzoic acid is 96.5%, the yield of m-toluic acid is 93.0%, p-toluic acid The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com