Protein of esterase EstK1 and application thereof
A technology of protein and esterase, applied in the direction of application, enzyme, hydrolase, etc., can solve the problems of low conversion rate and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
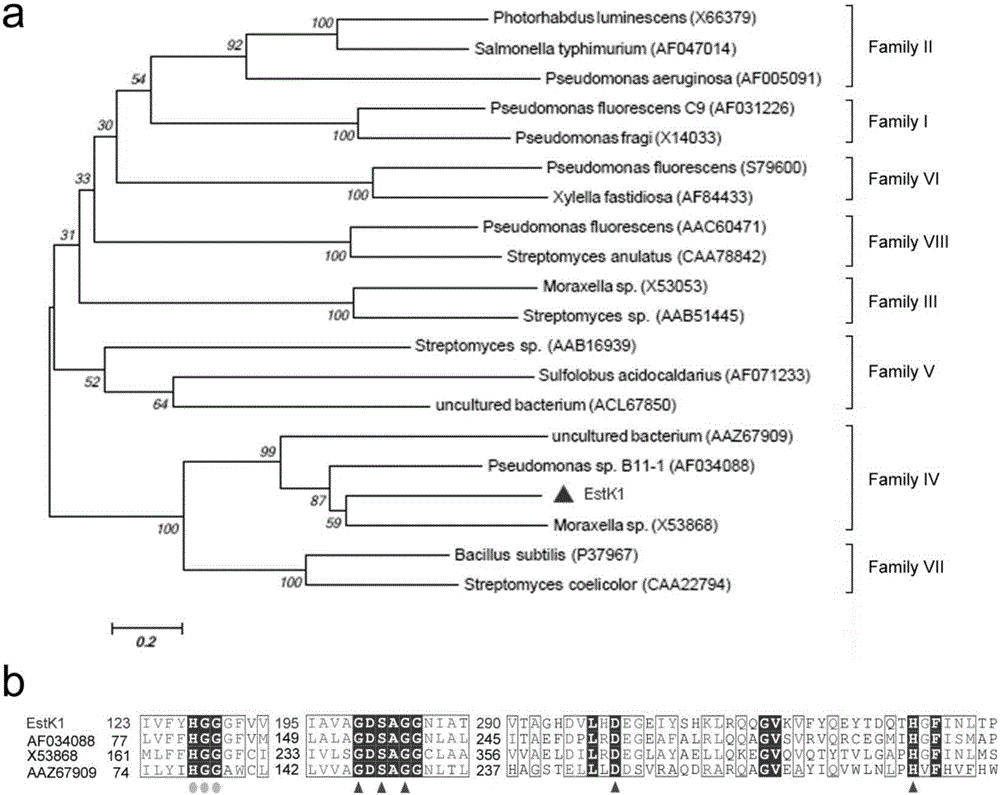
[0032] Embodiment 1: the construction of gene library and the analysis of lipolytic enzyme sequence
[0033] In the previous study, our laboratory screened a high-yielding esterase strain (OUC_Estk) and identified it as Acinetobacter haemolyticus. Genomic DNA of the bacterium was extracted, incompletely digested with Sau3AI restriction enzyme, and a 2-7kb DNA fragment was recovered. The recovered fragment was ligated into pBluescript IISK(+) vector which was completely digested with BamHI and dephosphorylated with alkaline phosphatase. The recombinant vector was introduced into DH5α competent cells, and spread on the LB ampicillin-resistant solid plate containing tributyrin. After standing in a 37°C incubator for 1-2 days, pick positive clones with obvious transparent circles for culture, extract plasmids and send them for sequencing. After the sequencing, through ORF prediction and BLASTP alignment, a lipolytic enzyme full-length gene with a size of 1071bp was obtained. Ph...
Embodiment 2
[0034] Example 2: Cloning, expression and purification of esterase EstK1
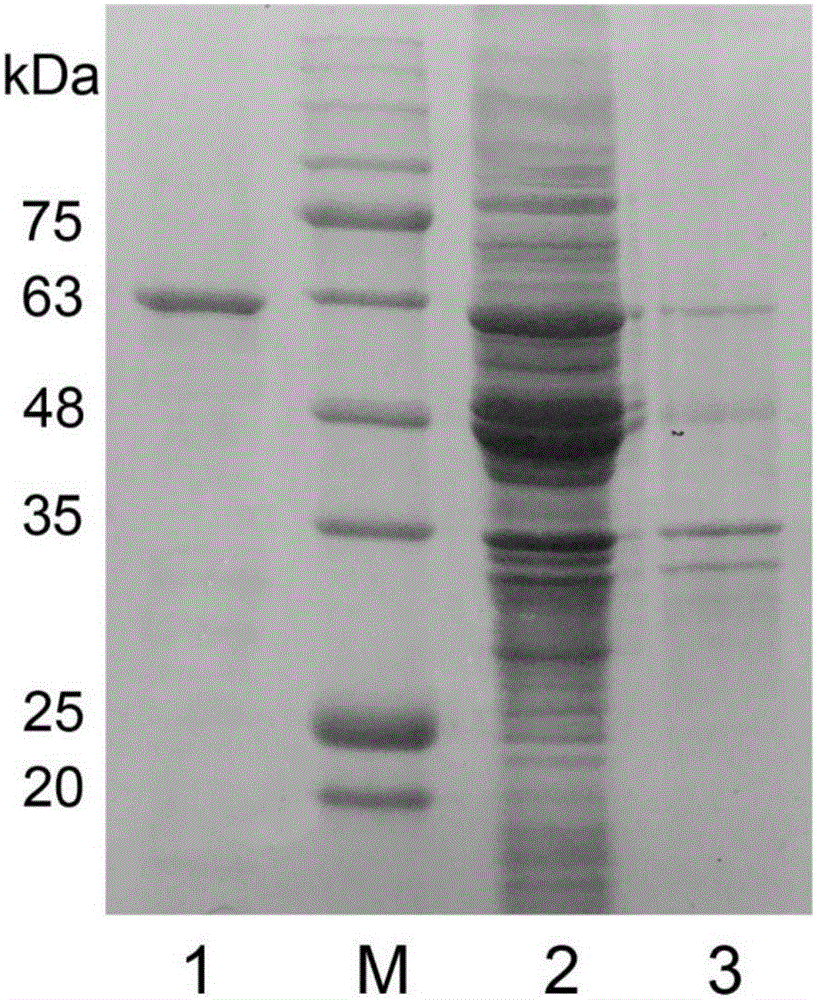
[0035] According to the obtained full-length EstK1 gene, design the full-length primer of the gene, upstream primer: CGC GGATCC ATGTTTGCATTGAATGAATCACTTA (BamHI restriction site), downstream primer: CCC AAGCTT TTAACTACGTTTATCCCAAAAATTTACG (HindIII restriction site). The digested target fragments were respectively ligated with pET21a(+), pET28a(+), pET32a(+) vectors cut with the same restriction enzymes, and the recombinant plasmids were finally introduced into BL21(DE3) for induced expression. After induction of expression, the bacteria were broken, and by enzyme activity detection, it was found that all three vectors could express active esterase. Because the protein content expressed by pET32a(+) was higher, pET32a(+) was selected as the final expression vector. . The recombinant protein EstK1 was purified.
[0036] The gel electrophoresis of the above-mentioned esterase EstK1 is as follows: f...
Embodiment 3
[0037] Embodiment 3: Research on the enzymatic properties of esterase EstK1
[0038] (1) Study on substrate specificity of esterase EstK1
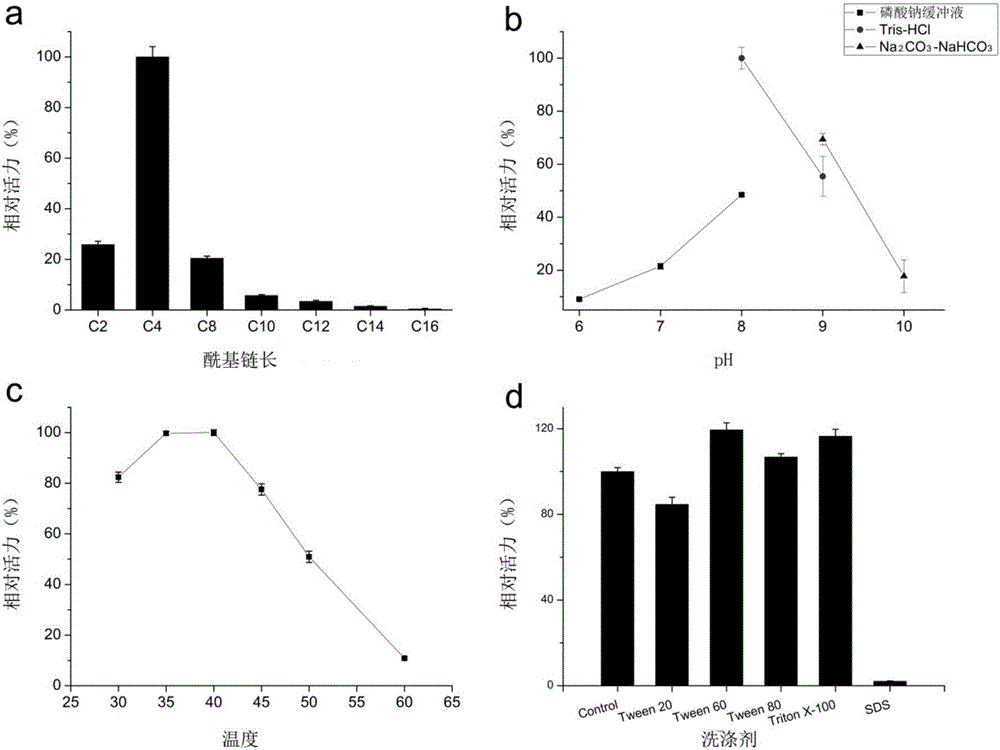
[0039] In 100mM pH 8.0 Tris-HCl buffer, pNP esters (C2, C4, C8, C10, C12, C14, C16) with different carbon chain lengths were used for detection to see the preference of esterase EstK1 for carbon chain length. Such as image 3 As shown in a, the esterase EstK1 shows the highest activity to C4, and the activity to pNP long-chain esters with >10 carbons is very low, indicating that the esterase EstK1 is an esterase.
[0040] (2) Optimum pH
[0041] The purified esterase EstK1 was tested using different pH buffers to detect the optimum pH of EstK1. The reaction process uses pNPB (C4) as the substrate, and the different pH buffers are: sodium phosphate buffer (pH 6, 7, 8), Tris-HCl buffer (pH 8, 9) and Na 2 CO 3 -NaHCO 3 Buffer (pH 9, 10). Such as image 3 As shown in b, the optimum pH of EstK1 is 8.0, and EstK1 shows higher activity in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com