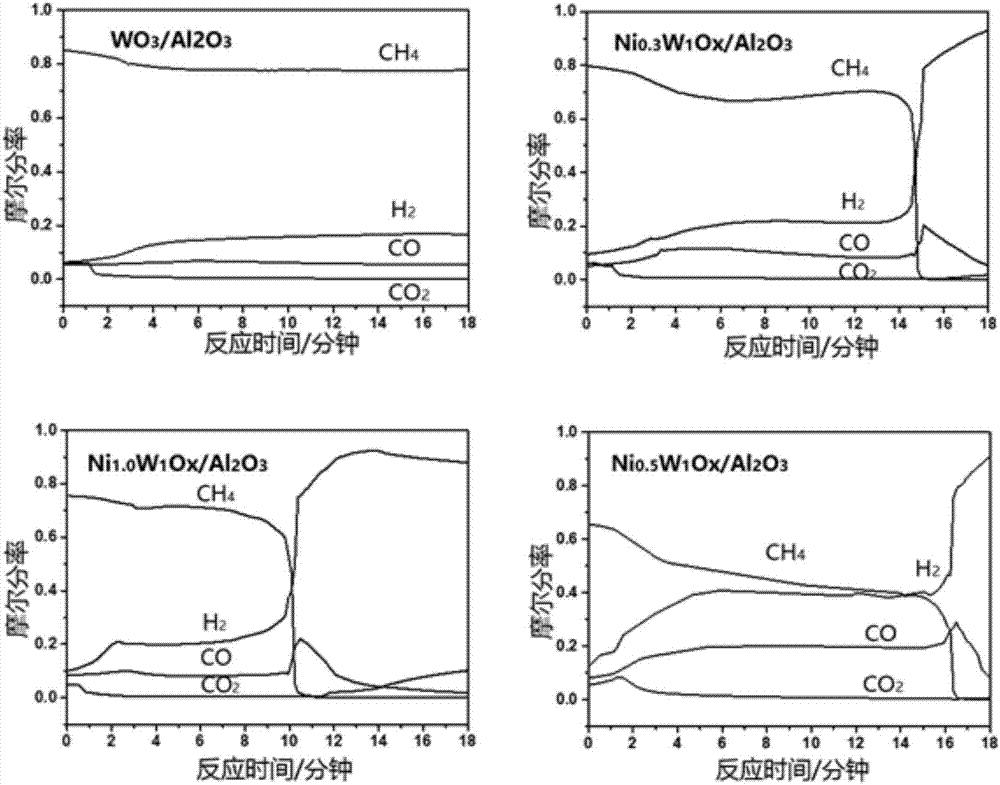
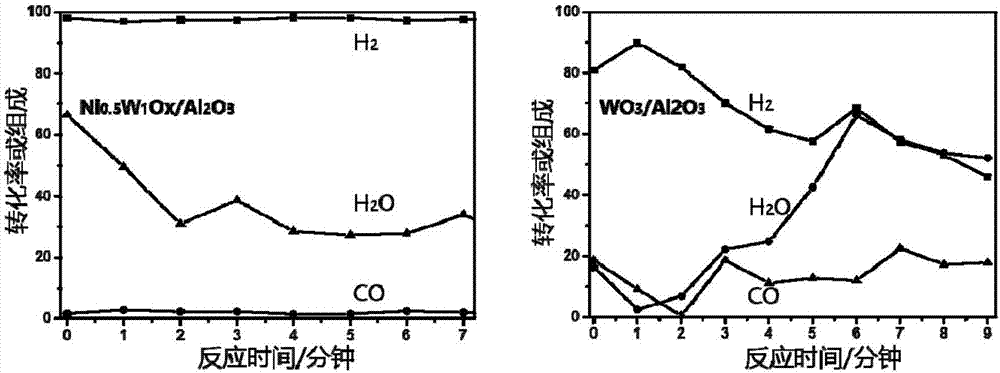
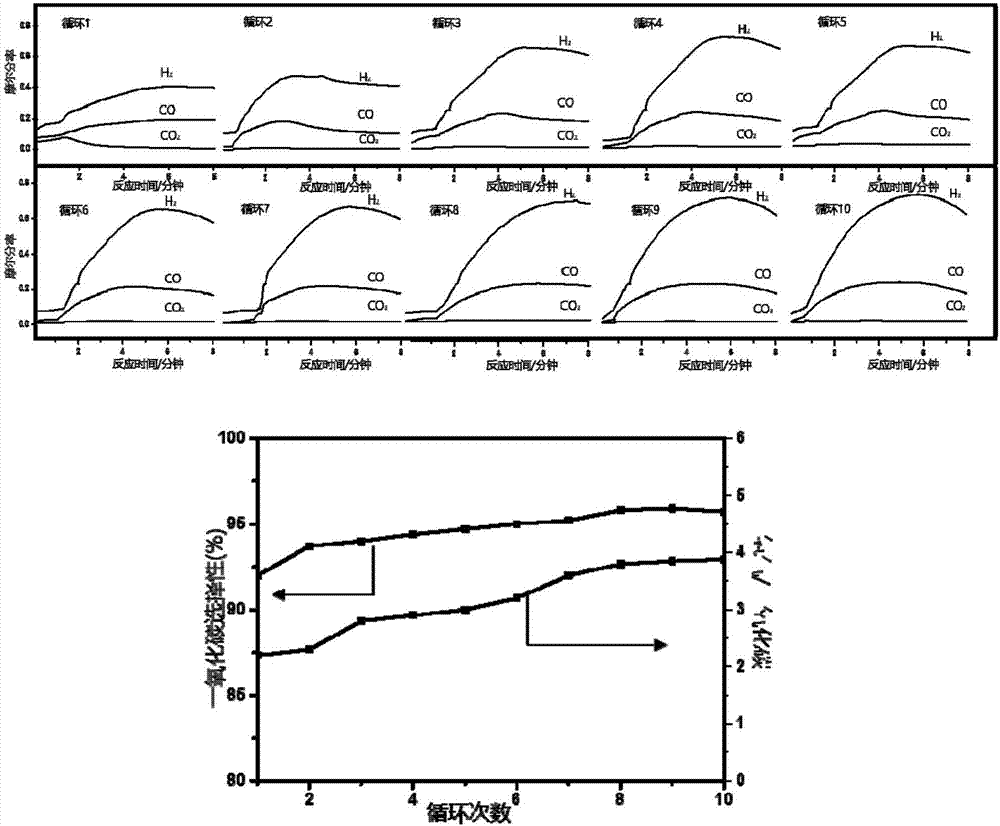
Supported nickel tungsten bimetallic composite oxide and preparation method and application thereof
A composite oxide and bimetallic technology, which is applied in the direction of metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, carbon compounds, etc., can solve the problems of low oxygen carrying capacity, complex preparation methods, Low hydrogen production efficiency and other problems, to achieve the effect of easy large-scale use, simple and easy preparation method, high hydrogen production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Weigh 1.9828g tungsten hexachloride (WCl 6 ), 12.7903g of aluminum nitrate nonahydrate (Al(NO 3 ) 3 9H 2 O) and 0.7270g of nickel nitrate hexahydrate (Ni(NO 3 ) 2 ·6H 2 O) dissolved in 50mL ethanol, ultrasonic 0.5h;
[0051] (2) Take 20g of NaOH and set the volume to 1M with a 500mL volumetric flask;
[0052] (3) Under the condition of magnetic stirring, slowly drop and mix the solution prepared in step 1 and step 2 at the same time until the pH is 9-10, and let the obtained flocculent precipitate stand at room temperature for 1 hour;
[0053] (4) Centrifuge, filter, and wash the flocculent precipitate in (3) with ethanol and distilled water successively for 5-6 times until the pH is 7-8;
[0054] (5) Dry the material obtained in (4) at room temperature for 12 hours, then dry it at 70-90°C for 12 hours, and finally bake it in an air atmosphere at 800°C for 4 hours to obtain a nickel-tungsten bimetallic composite oxide supported on alumina , whose molecular f...
Embodiment 2
[0056] (1) Weigh 1.9828g tungsten hexachloride (WCl 6 ) and 12.7903g of aluminum nitrate nonahydrate (Al(NO 3 ) 3 9H 2 O) dissolved in 50mL ethanol, ultrasonic 0.5h;
[0057] (2) Take 20g of NaOH and set the volume to 1M with a 500mL volumetric flask;
[0058] (3) Under the condition of magnetic stirring, slowly drop and mix the solution prepared in step 1 and step 2 at the same time until the pH is 9-10, and let the obtained flocculent precipitate stand at room temperature for 1 hour;
[0059] (4) Centrifuge, filter, and wash the flocculent precipitate in (3) with ethanol and distilled water successively for 5-6 times until the pH is 7-8;
[0060] (5) Dry the material obtained in (4) at room temperature for 12 hours, then dry it at 70-90°C for 12 hours, and finally bake it in an air atmosphere at 800°C for 4 hours to obtain tungsten oxide supported on alumina, whose molecular formula is WO 3 / Al 2 o 3 .
Embodiment 3
[0062] (1) Weigh 1.9828g tungsten hexachloride (WCl 6 ), 12.7903g of aluminum nitrate nonahydrate (Al(NO 3 ) 3 9H 2 O) and 0.4847 nickel nitrate hexahydrate (Ni(NO 3 ) 2 ·6H 2 O) dissolved in 50mL ethanol, ultrasonic 0.5h;
[0063] (2) Take 20g of NaOH and set the volume to 0.5M with a 500mL volumetric flask;
[0064] (3) Under the condition of magnetic stirring, the solution prepared in step 1 and step 2 was slowly added dropwise and mixed at the same time until the pH was 9-10, and the obtained flocculent precipitate was left to stand at room temperature for 2 hours;
[0065] (4) Centrifuge, filter, and wash the flocculent precipitate in (3) with ethanol and distilled water successively for 5-6 times until the pH is 7-8;
[0066] (5) Dry the material obtained in (4) at room temperature for 8 hours, then dry it at 70-90°C for 8 hours, and finally bake it in an air atmosphere at 600°C for 5 hours to obtain a nickel-tungsten double metal composite oxide supported on alum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com