Preparation method of polyester
A polyester and cyclic lactone technology, applied in the fields of organic catalysis and polymer materials, can solve the problems of catalyst recovery, high cost, and inability to simultaneously reflect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
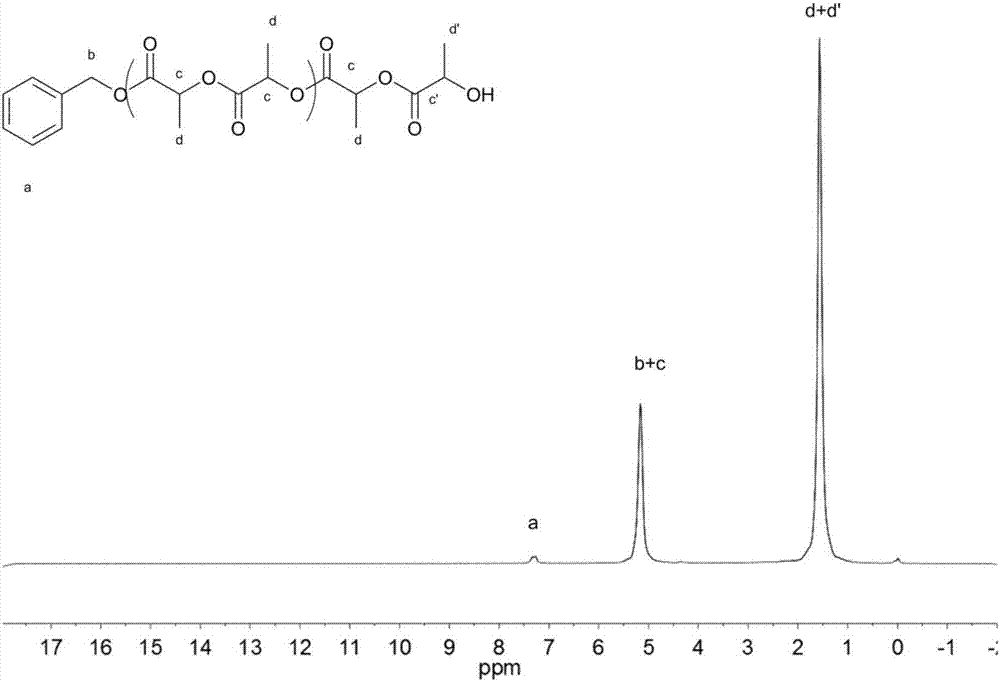
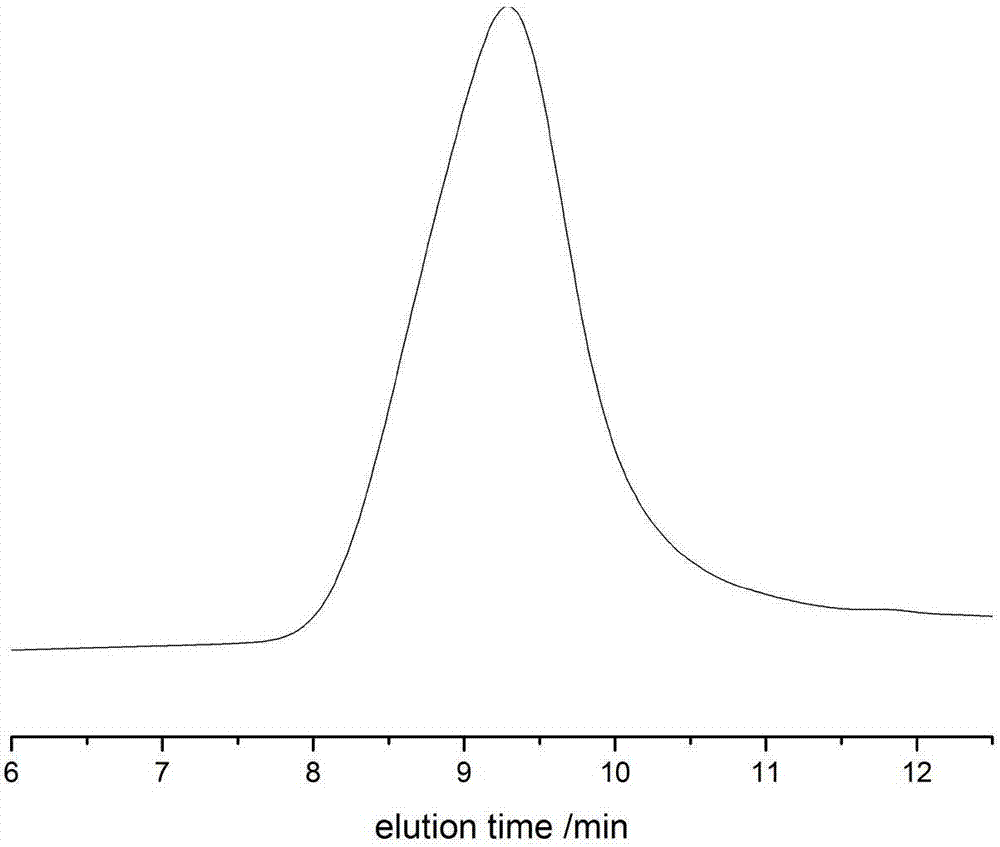
[0080] In a 10 mL polymerization tube, add butyrolactone (0.3856 g, 2.88 mmol), benzyl alcohol (10 μl, 0.096 mmol), and the compound shown in No. 13 (0.029 g, 0.096 mmol), and stir magnetically at 90 ° C for 2 h . After the reaction, the obtained crude product was dissolved in a minimum amount of dichloromethane, and then added into a cold methanol solution, and a polymer was precipitated. Centrifugation obtained 0.23 g of white solid, which was transferred to a vacuum drying oven for drying. polymer structure through 1 H NMR with 13 C NMR identification, the molecular weight and dispersion of the polymer were determined by GPC. After determination, the conversion rate of polymer is 90.6%, and the number average molecular weight M n 2600g mol -1 , M n / M w is 1.36. The compound shown in No. 13 of the present invention, in a 250ml reaction bottle, add the compound shown in No. 1 (12.27mmol, 1.5g), saccharin (12.27mmol, 1.5g), and stir overnight at 60°C in tetrahydrofura...
Embodiment 2
[0082] In a 10 mL polymerization tube, add valerolactone (0.288 g, 2.88 mmol), benzyl alcohol (10 microliters, 0.096 mmol), and the compound shown in No. 14 (0.0375 g, 0.096 mmol), and stir magnetically at 90 ° C for 1 h . After the reaction, the obtained crude product was dissolved in a minimum amount of dichloromethane, and then added into a cold methanol solution, and a polymer was precipitated. Centrifugation obtained 0.21 g of white solid, which was transferred to a vacuum drying oven for drying. polymer structure through 1 H NMR with 13 C NMR identification, the molecular weight and dispersion of the polymer were determined by GPC. After determination, the transformation rate of polymer is 92.8%, and the number average molecular weight M n 2900g mol -1 , M n / M w is 1.21. The compound shown in No. 14 of the present invention, in a 250ml reaction bottle, add the compound shown in No. 2 (12.27mmol, 1.5g), saccharin (12.27mmol, 1.5g), and stir overnight at 60°C in t...
Embodiment 3
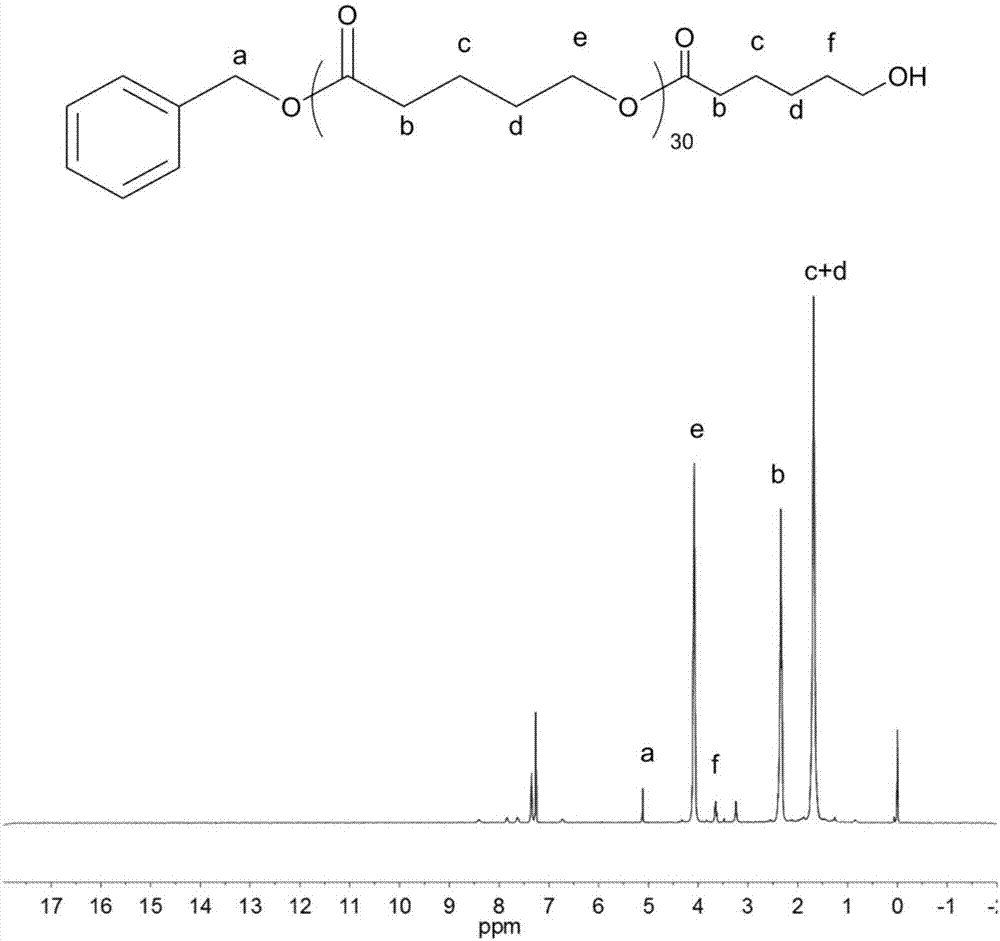
[0084]In a 10 mL polymerization tube, add caprolactone (0.328 g, 2.88 mmol), benzyl alcohol (10 microliters, 0.096 mmol), and the compound shown in No. 16 (0.038 g, 0.096 mmol), and stir magnetically at 100 ° C for 1 h . After the reaction, the obtained crude product was dissolved in a minimum amount of dichloromethane, and then added into a cold methanol solution, and a polymer was precipitated. Centrifugation obtained 0.23 g of white solid, which was transferred to a vacuum drying oven for drying. polymer structure through 1 H NMR with 13 C NMR identification, the molecular weight and dispersion of the polymer were determined by GPC. After determination, the transformation rate of polymer is 98.7%, and the number average molecular weight M n 3500g mol -1 ,M n / M w is 1.37. The compound shown in No. 16 of the present invention, in a 250ml reaction bottle, add the compound shown in No. 4 (12.27mmol, 1.5g), saccharin (12.27mmol, 1.5g), and stir overnight at 60°C in tetr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com