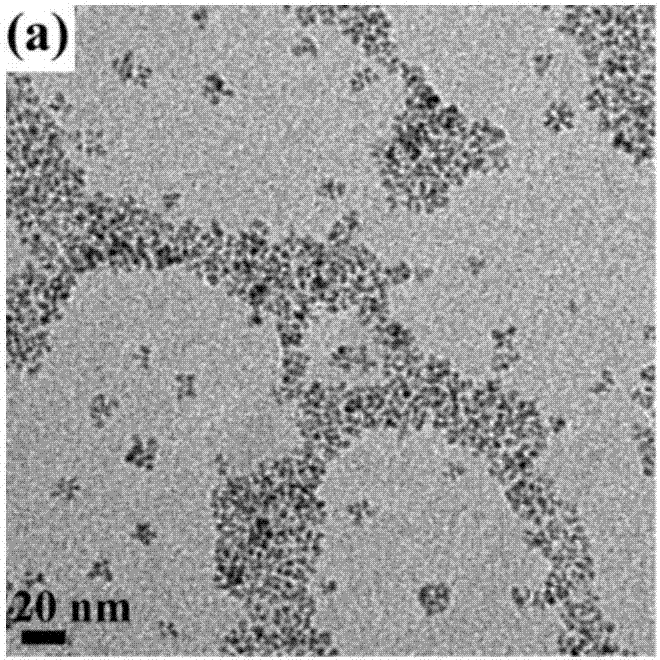
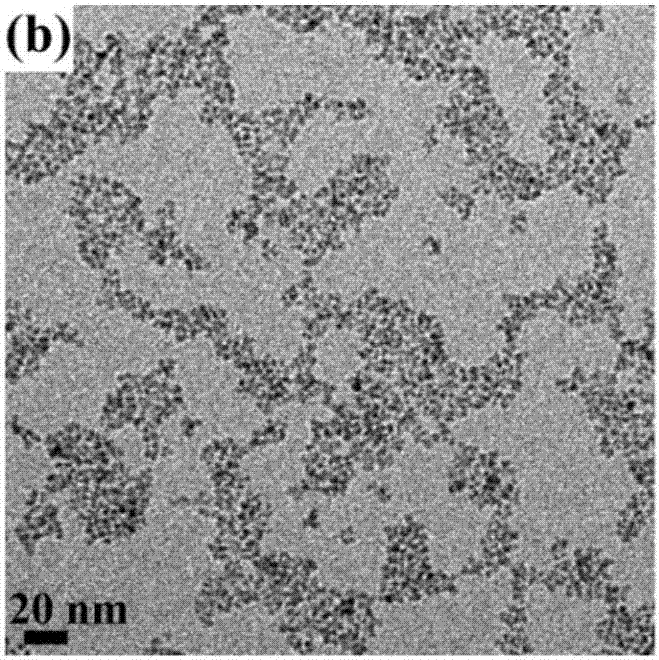
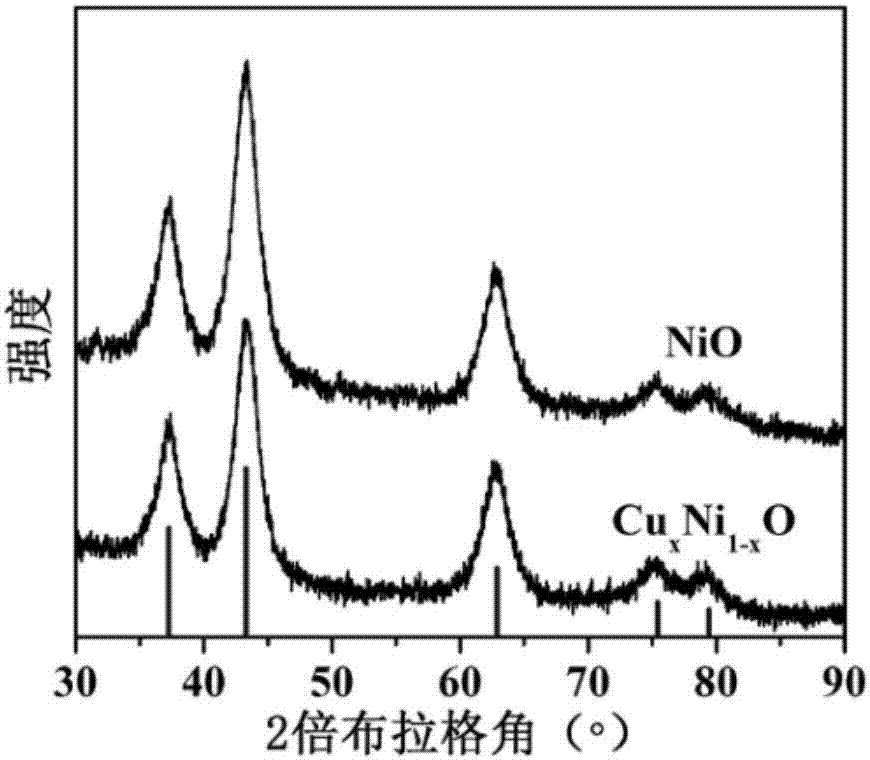
Copper ion-doped nickel oxide colloidal nano-crystal preparation method and product thereof, and applications of copper ion-doped nickel oxide colloidal nano-crystal
A nickel oxide and nanocrystalline technology, applied in the preparation of nickel compounds, nickel oxide/hydroxide, nanotechnology, etc., can solve the problems of limited application and incompatibility with high temperature resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] A preparation method for copper ion-doped nickel oxide colloidal nanocrystals, comprising the following steps:
[0095] (1) Synthesis of metal precursors
[0096] 1) Synthesis of nickel isobutyrate (nickelisobutyrate, Ni(iBu) 2 ):
[0097] Weigh 20mmol (1.7800g) of isobutyric acid (liquid) and dissolve it in 30g of anhydrous methanol to obtain an isobutyric acid solution; another weigh 20mmol (3.6986g) of tetramethylammonium hydroxide pentahydrate and dissolve it in 10g of anhydrous In water and methanol, a tetramethylammonium hydroxide solution was obtained; the two solutions were mixed and stirred for 20 minutes, and acid-base neutralization reaction was carried out to obtain solution I;
[0098] Then weigh 10mmol (2.9673g) of nickel nitrate hexahydrate, dissolve it in 10g of anhydrous methanol to obtain solution II; add solution II dropwise to solution I, and keep stirring for 30min to fully proceed the reaction to obtain solution III ;
[0099] After adding exce...
Embodiment 2
[0111] A preparation method for copper ion-doped nickel oxide colloidal nanocrystals, comprising the following steps:
[0112] (1) Synthesis of metal precursors
[0113] 1) Synthesis of nickel 2-ethylhexanoate:
[0114] Nickel iso-octanoate was synthesized in the same manner as in Example 1 "1) Synthesis of nickel isobutyrate, except that iso-octanoic acid was used instead of isobutyric acid.
[0115] 2) Synthesis of copper 2-ethylhexanoate
[0116] Copper isooctanoate was synthesized in the same manner as in Example 1 "2) Synthesis of copper isobutyrate, except that isooctanoic acid was used instead of isobutyric acid.
[0117] (2) Synthesis of copper ion-doped nickel oxide colloidal nanocrystals (Cu x Ni 1-x O nanocrystal)
[0118] 1) Synthesis of nickel oxide nanocrystals:
[0119] The synthesis of nickel oxide nanocrystals was carried out on a Schlenk device, as follows: 0.5 mmol of nickel isooctanoate, 0.2 mmol of potassium stearate, 3 mmol of dodecyl alcohol and 5 ...
Embodiment 3
[0126] A preparation method for copper ion-doped nickel oxide colloidal nanocrystals, comprising the following steps:
[0127] (1) Synthesis of metal precursors
[0128] 1) 1) Synthesis of nickel isobutyrate (nickelisobutyrate, Ni(iBu) 2 ):
[0129] Same as Example 1.
[0130] 2) Synthesis of copper 2-ethylhexanoate
[0131] Same as Example 2.
[0132] (2) Synthesis of copper ion-doped nickel oxide colloidal nanocrystals (Cu x Ni 1-x O nanocrystal)
[0133] 1) Synthesis of nickel oxide nanocrystals:
[0134] The synthesis of nickel oxide nanocrystals is carried out on the Schlenk device, specifically as follows: 0.4mmol of Ni(iBu) 2 , 0.2mmol of sodium stearate, 3mmol of trioctylamine and 5mL of diphenyl ether were placed in a 25mL round-bottomed flask; argon gas was passed through the flask for 10 minutes, then the temperature was raised, and vacuum was drawn at 100 and 120°C for 30 minutes respectively Re-introduce argon gas, directly raise the temperature of the re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Work function | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com