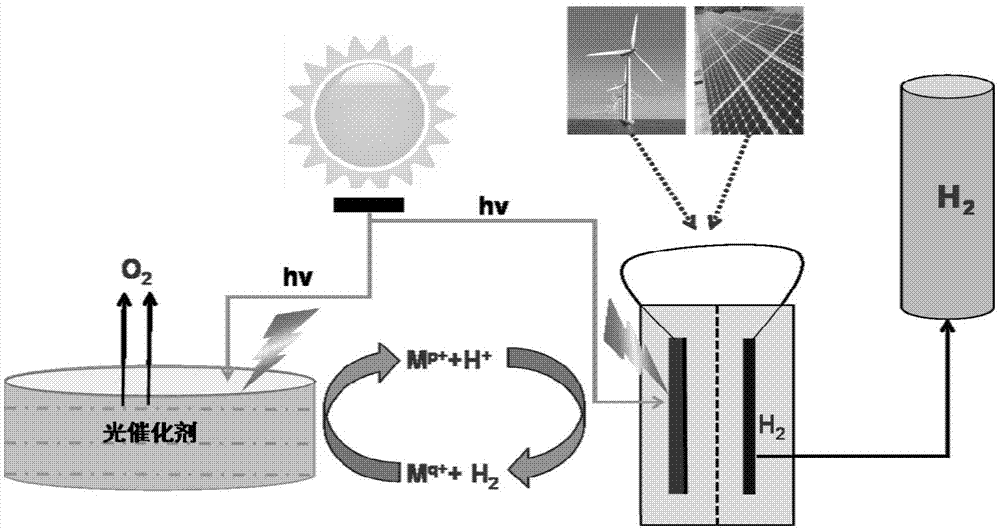
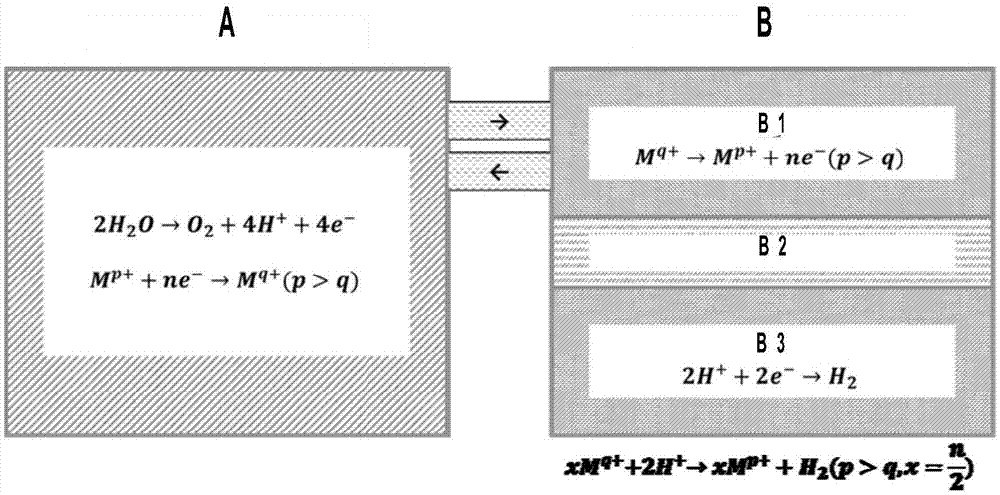
Large-scale solar water decomposition hydrogen production method based on photocatalysis - photoelectrocatalysis
A photocatalytic and photocatalytic technology, which is applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of limited utilization of the solar spectrum of catalysts, insufficient efficiency, and safety Hidden dangers and other problems, to achieve the effect of promoting the separation of photogenerated charges, solving difficulties in storage, and good crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
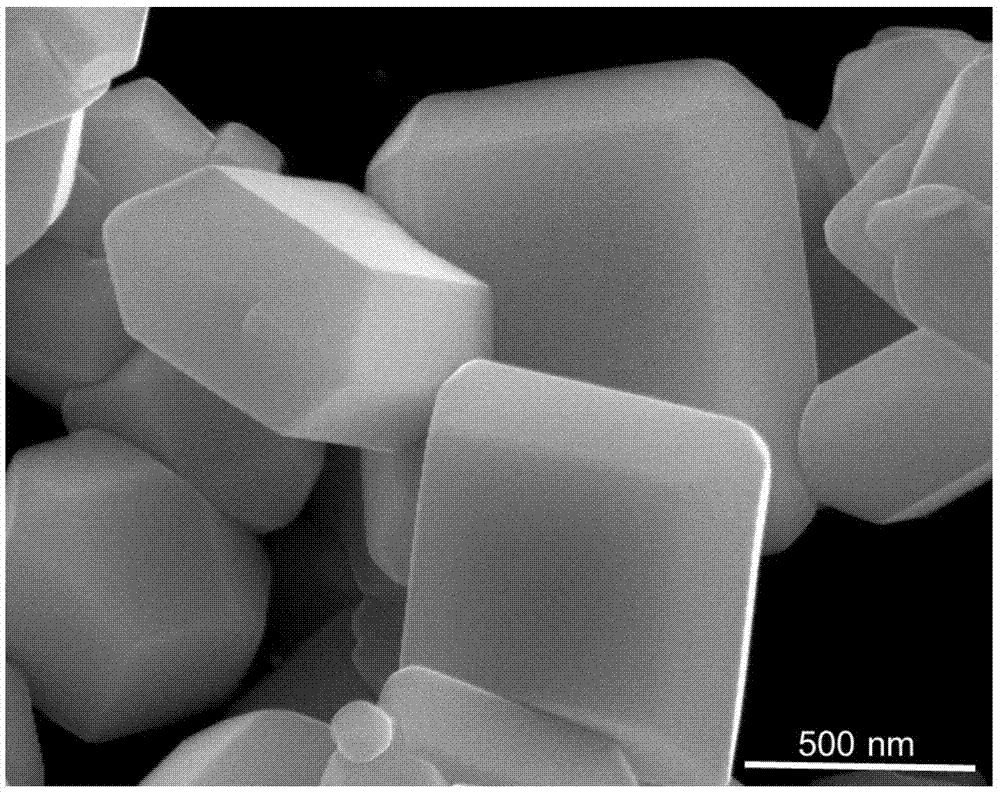
[0056] With the photocatalyst BiVO described in the present invention 4 As an example, hydrothermal synthesis and deposition precipitation are used for synthesis. The synthesis process is as follows: respectively dissolve bismuth source (100mM) and vanadium source (100mM) in acid solution, mix them evenly at a volume ratio of 1:1, and add structure-oriented (0.05mM) solution, gradually adjust the pH value with ammonia solution, stir, then transfer to a round bottom flask at a certain temperature under normal pressure and keep it for a certain period of time. After the reaction, centrifuge, wash and dry in an oven. Wherein, the concentration of the bismuth source and the vanadium source is 1-1000mM (100mM here), the concentration of the acid solution is 0.1-15M (1M here), and the concentration of the structure-directing agent solution is 0.1-10mM (1mM here). ), the pH value is adjusted to 0-10 (here it is 5), the stirring time is 0.1-5h (here it is 3h), the reaction temperature...
Embodiment 2
[0059] Performance Evaluation of Photocatalysts in Water Oxidation in the Presence of Different Electron Carriers
[0060] 100mg of photocatalyst was dispersed in 150mL of different electron carrier solutions (10mM), and then the reaction system was evacuated, under the condition of 300W xenon lamp (λ>420nm) light, after reaction for 1h, samples were taken for analysis, and the gas phase products obtained from the reaction were detected online by gas chromatography , by detecting the amount of oxygen generated by the oxidation reaction to calculate the conversion rate of the high-valence electron carrier, the results are listed in Table 1.
[0061] Table 1. Reactivity of photocatalysts in the presence of different electron carriers
[0062]
[0063]
Embodiment 3
[0065] Photocatalyst in Fe 3+ Time-dependent evaluation of photocatalytic water oxidation reaction in ionic system (based on BiVO 4 Photocatalyst as an example)
[0066] 100mg photocatalyst BiVO 4 dispersed in Fe 3+ solution (150mL), then evacuate the reaction system, under the condition of 300W xenon lamp (λ>420nm) light, after a certain period of time, take a sample for analysis, and the gas phase product obtained by the reaction is detected online by gas chromatography. Fe 3+ Two solutions of 3.5mM and 10.0mM were selected respectively, and the reaction can make Fe 3+ Complete conversion of Fe 2+ , generating stoichiometric O 2 , which is in full agreement with the theoretical value, proving that the photocatalyst can completely convert Fe 3+ to Fe 2+ , that is, there is no reverse reaction, and the obtained data are as follows Figure 5 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com