Alpha-benzyl-modified diaryl phosphonic compound and preparation method thereof
A technology of aryl phosphines and compounds, applied in the field of organic synthesis, to achieve the effect of wide application range and high selectivity of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
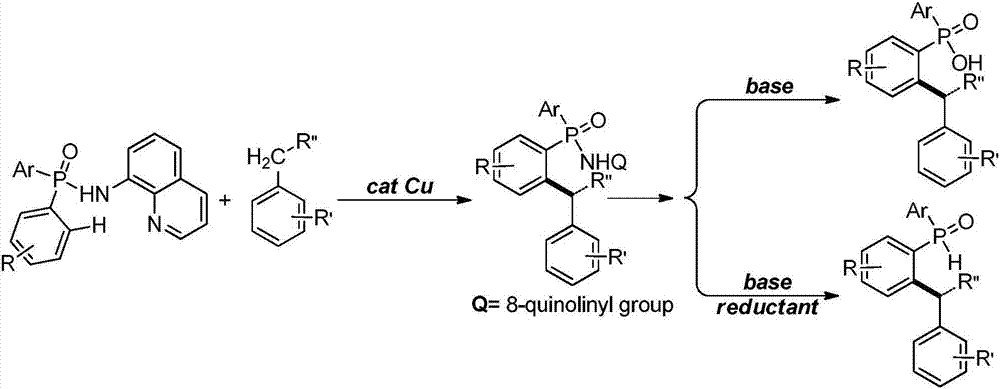
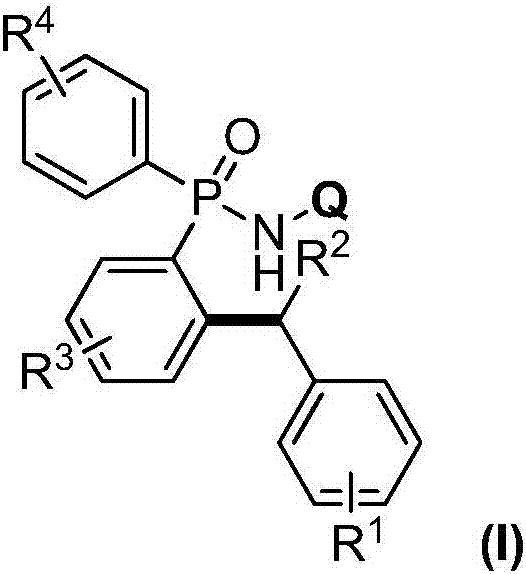
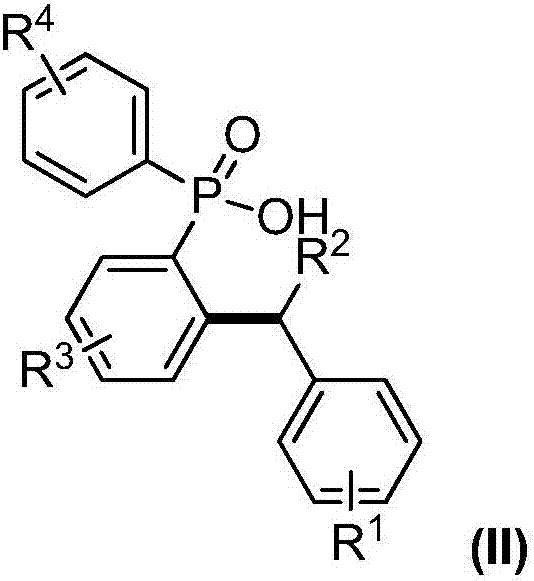
[0032] Add 0.005mmol copper powder and 0.1mmol P,P-diphenyl-N-(8-aminoquinoline)-phosphonamide into a 25mL Schlenk reaction tube, vacuum backfill nitrogen 5 times; under nitrogen atmosphere, add toluene in sequence and di-tert-butyl peroxide (DTBP), continue to react at 100°C for 24h; after the reaction is completed, cool to room temperature and separate by column chromatography to obtain the target product I; the reaction result is: P-(2-benzylphenyl )-P-phenyl-N-(8-aminoquinoline)-phosphonamide, the productive rate is 93%; Get P-(2-benzylphenyl)-P-phenyl-N-(8-aminoquinoline Phenyl)-phosphonamide was placed in a 25mL Schlenk reaction tube, followed by adding 2mol / L aqueous sodium hydroxide solution and ethanol, and reacted vigorously at 80°C for 2h; after the reaction was completed, it was cooled to room temperature and separated by column chromatography to obtain the target product P-(2-benzylphenyl)-P-phenyl-N-(8-aminoquinoline)-phosphonic acid, the yield is 92%; take P-(2-...
preparation example 2
[0034] Add 0.01mmol cuprous oxide and 0.1mmol P,P-diphenyl-N-(8-aminoquinoline)-phosphonamide into a 25mL Schlenk reaction tube, vacuum backfill nitrogen 5 times; under nitrogen atmosphere, add P-xylene and tert-butyl hydroperoxide (TBHP), continue to react at 100°C for 24h; after the reaction is completed, cool to room temperature and separate by column chromatography to obtain the target product I; the reaction result is: P-[2-( 4-methylbenzyl) phenyl]-P-phenyl-N-(8-aminoquinoline)-phosphonamide, the productive rate is 98%; P-[2-(4-methylbenzyl)benzene Base]-P-phenyl-N-(8-aminoquinoline)-phosphonamide was placed in a 25mL Schlenk reaction tube, and 2mol / L sodium hydroxide aqueous solution and ethanol were added successively, and reacted violently at 80°C for 2h; Cool to room temperature after completion, and separate by column chromatography to obtain the target product P-[2-(4-methylbenzyl)phenyl]-P-phenyl-N-(8-aminoquinoline)-phosphonic acid, The yield rate is 90%; P-[2-(...
preparation example 3
[0036]Add 0.015mmol copper chloride, 0.1mmol P,P-diphenyl-N-(8-aminoquinoline)-phosphonamide into a 25mL Schlenk reaction tube, vacuum backfill nitrogen 5 times; under nitrogen atmosphere, add Toluene and hydrogen peroxide continued to react at 100°C for 24 hours; after the reaction was completed, cooled to room temperature and separated by column chromatography to obtain the target product I; the reaction result was: P-[2-(3-methylbenzyl)phenyl] -P-phenyl-N-(8-aminoquinoline)-phosphonamide, the productive rate is 99%; Take P-[2-(4-methylbenzyl) phenyl]-P-phenyl-N- (8-Aminoquinoline)-phosphonamide was placed in a 25mL Schlenk reaction tube, followed by adding 2mol / L sodium hydroxide aqueous solution and ethanol, and reacted vigorously at 80°C for 2h; after the reaction was completed, cooled to room temperature and separated by column chromatography , to obtain the target product P-[2-(3-methylbenzyl) phenyl]-P-phenyl-N-(8-aminoquinoline)-phosphonic acid, the productive rate is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com