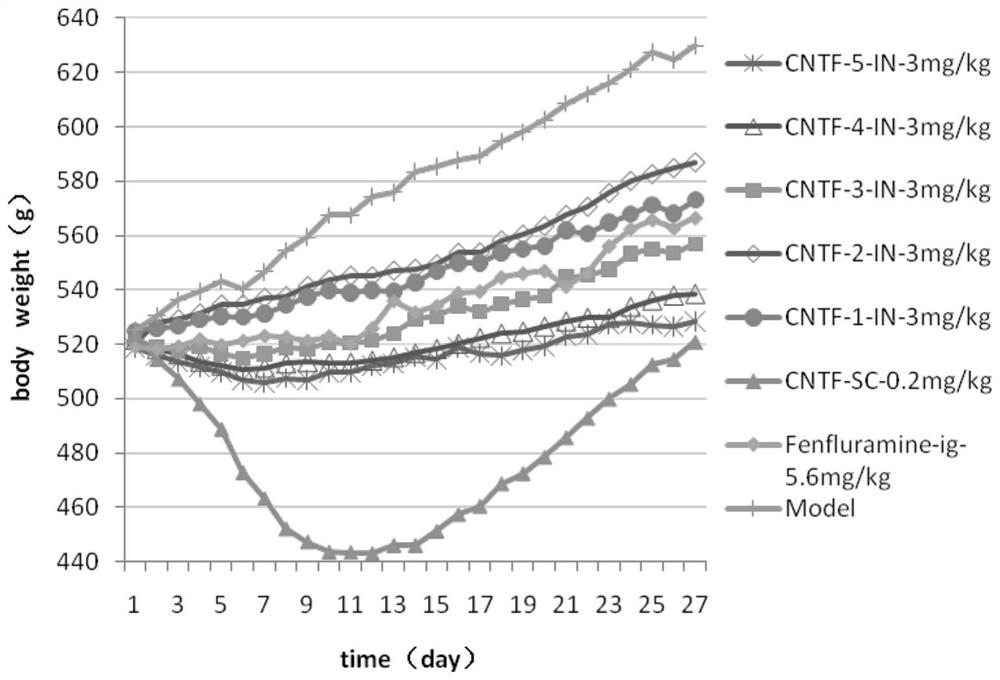
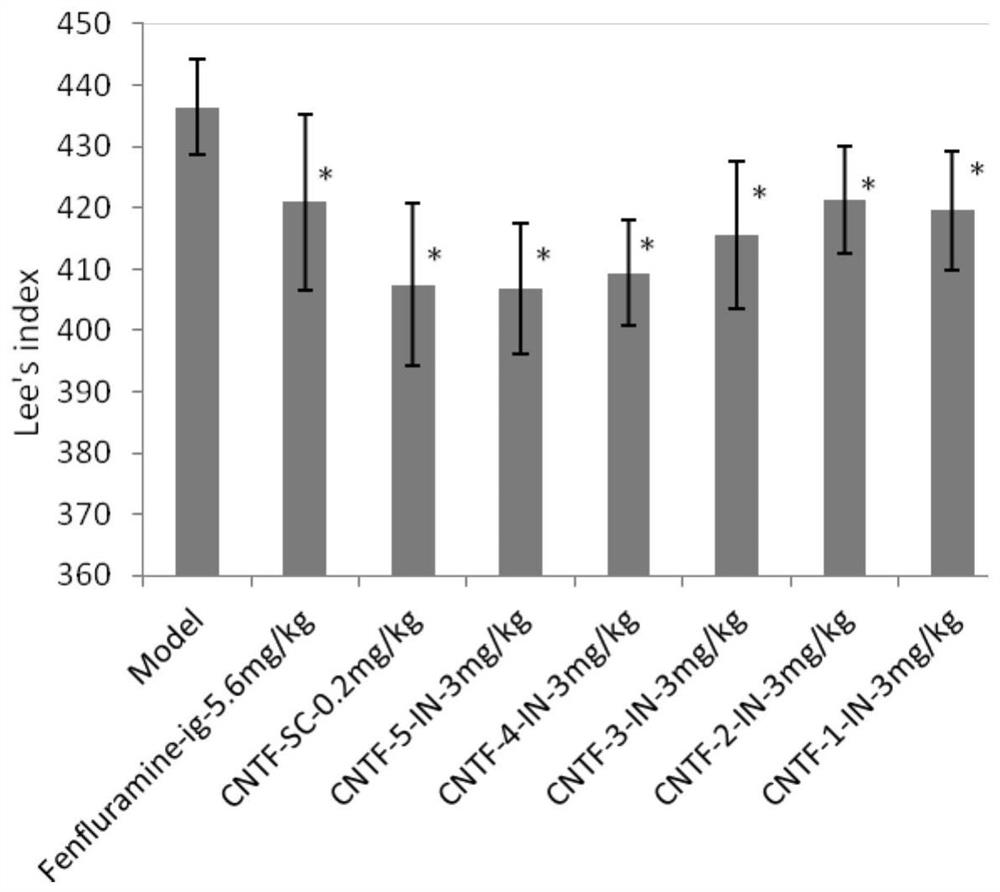
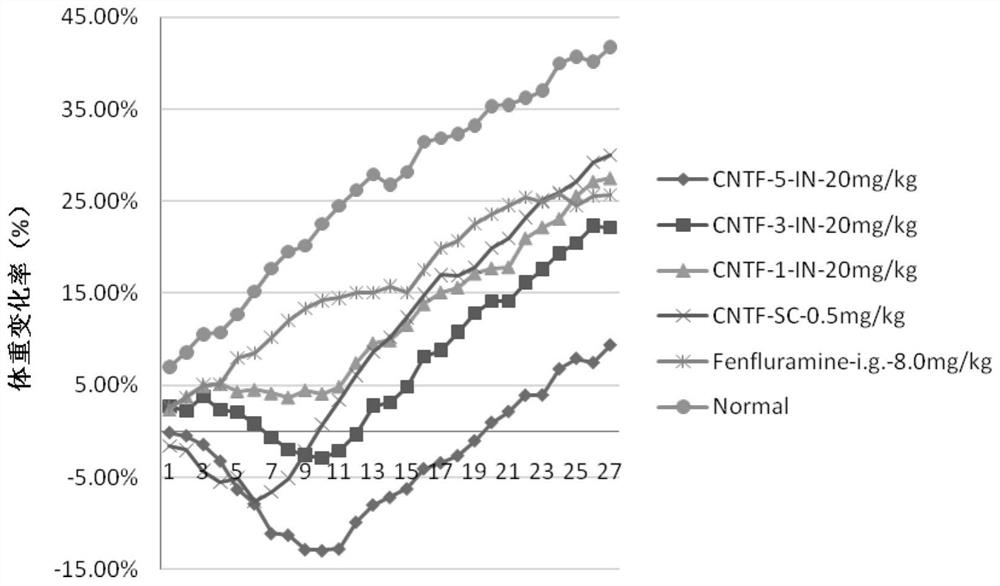
Ciliary neurotrophic factor nasal drug delivery system and its preparation method and application
A technology for ciliary neurotrophic and nasal administration preparations, applied in the field of medicine, can solve the problems of prolonging the half-life of CNTF, increasing protein stability, impermeability of the blood-brain barrier, etc., and achieving the effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: CNTF nasal drops
[0044] 1. Composition: CNTF 0.015g, hydroxypropyl-β-cyclodextrin 25g, mannitol 0.1g and PBS buffer (pH 7.4) 100ml.
[0045] 2. Preparation method:
[0046] 1) PBS buffer preparation method: weigh 2.457g of sodium dihydrogen phosphate and dissolve it in 50ml of water to make 0.315M solution A; weigh 5.6405g of disodium hydrogen phosphate and dissolve it in 20ml of water to make solution B; B liquid to PH = 7.4; B liquid volume to 50ml. When used, take 10ml and add 0.45g sodium chloride to dilute to 50ml, the concentration is 0.063M, and set aside.
[0047] 2) Treatment of CNTF: take 32ml of CNTF stock solution (provided by China Institute for the Control of Pharmaceutical and Biological Products in Beijing, the same below) and place it in the liquid storage tube of the ultrafiltration centrifugal device, divide it into three times, centrifuge at 6500r at 4°C for 30min; add distilled water to the filtrate 10ml, centrifuge for 30min, and...
Embodiment 2
[0049] Embodiment 2: CNTF nasal drops
[0050] 1. Composition: CNTF 0.015g, polysorbate 20 10g, lactose 0.1g and PBS buffer (pH7.4) 100ml.
[0051] 2. Preparation process:
[0052] PBS damping fluid and CNTF processing method are the same as embodiment 1;
[0053] Dissolve the polysorbate and lactose in the formula amount in PBS buffer solution, place in the bottle of the nasal drug delivery device, then put CNTF in the storage rack of the nasal drug delivery device, cover the bottle cap, and set aside.
Embodiment 3
[0054] Embodiment 3: CNTF nasal drops
[0055] 1. Composition: CNTF 0.030g, polyethylene glycol stearate 15 14.29g, lactose 0.1g, PBS buffer (pH7.4) 100ml.
[0056] 2. Preparation process:
[0057] PBS damping fluid and CNTF processing method are the same as embodiment 1;
[0058] The polyethylene glycol stearate 15 of formula quantity, lactose are dissolved in the PBS damping fluid, are placed in the bottle body of nasal applicator, then CNTF is placed in the storage rack of nasal applicator, cover Good bottle cap, spare.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com