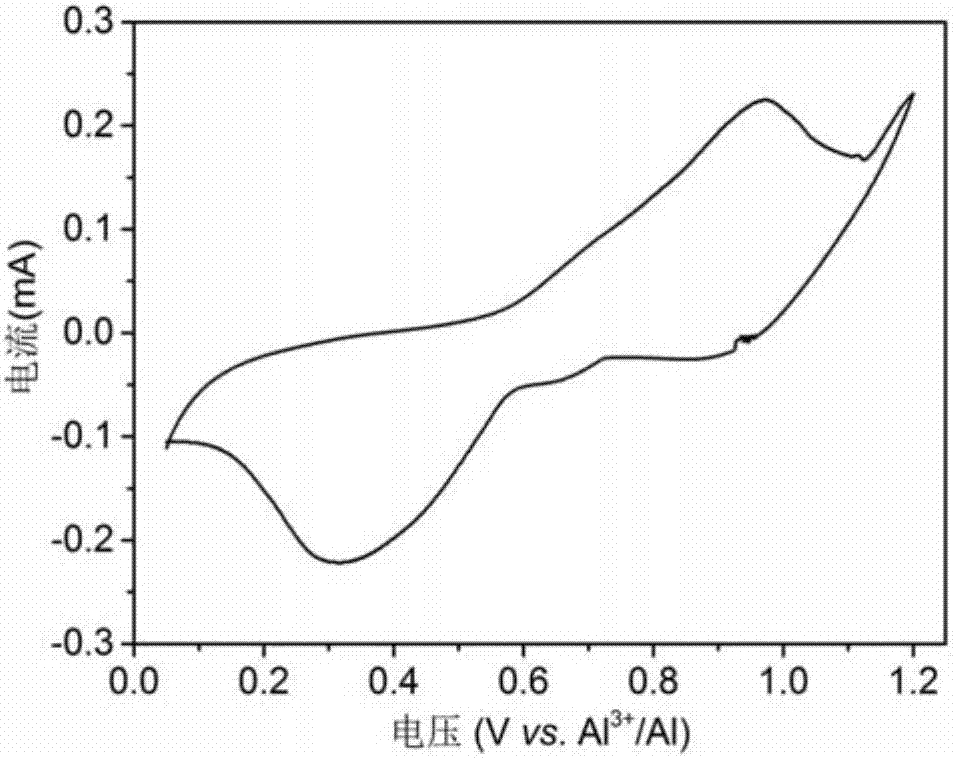
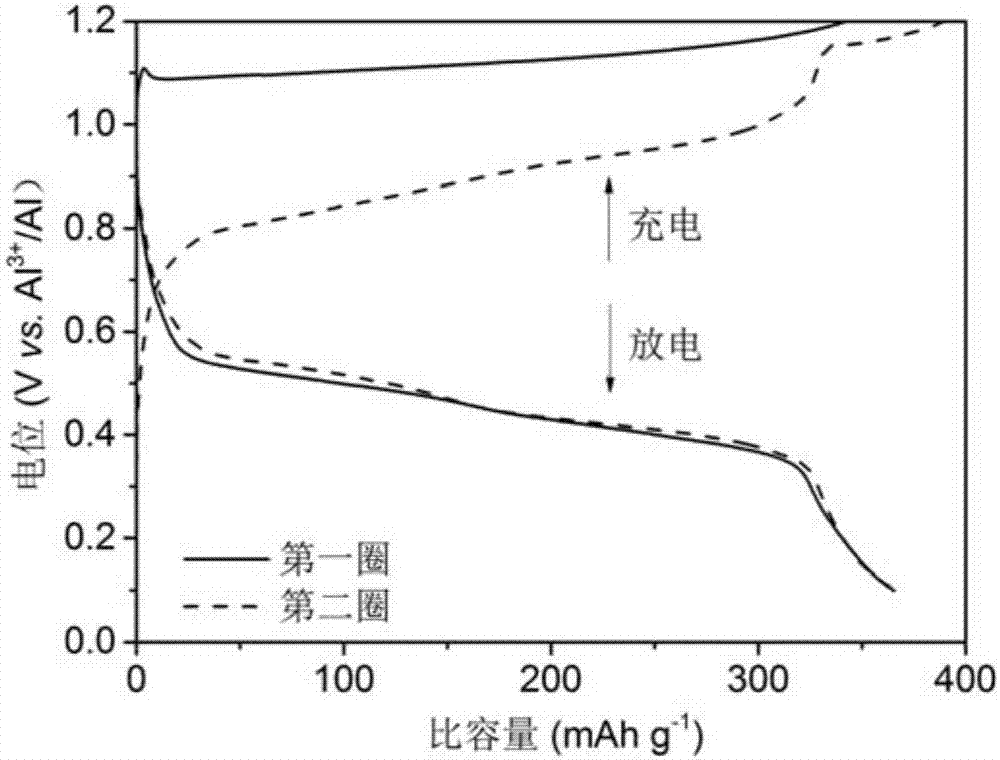
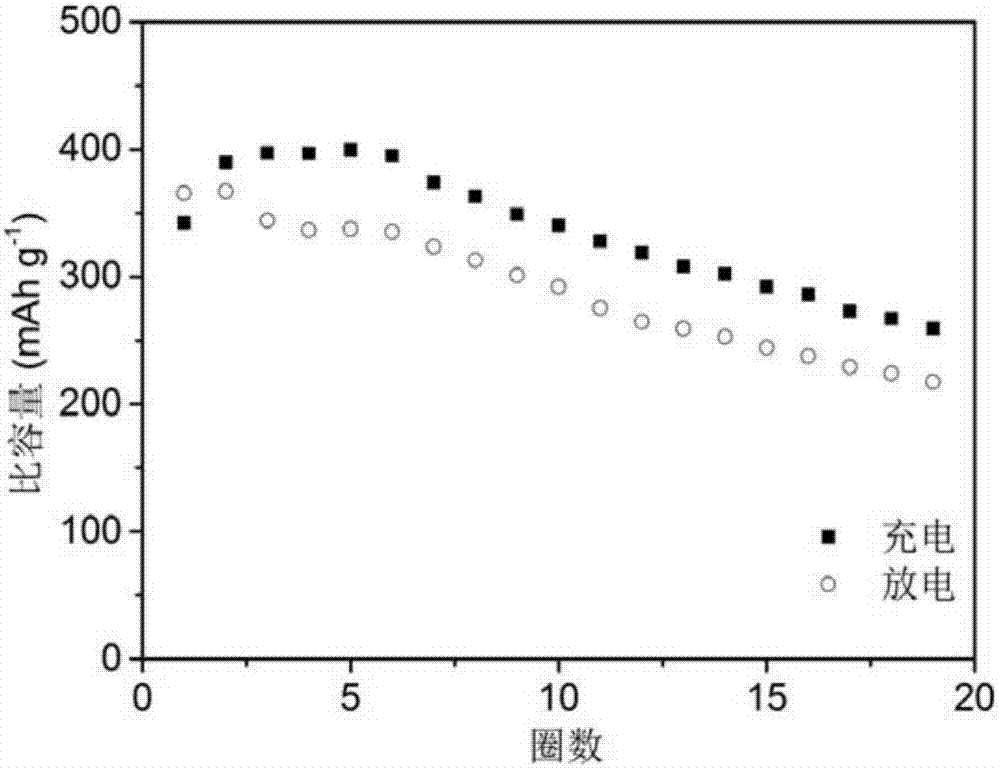
Rechargeable-dischargeable aluminum ion battery taking copper-selenium compound as positive electrode
A copper-selenium compound, aluminum-ion battery technology, applied in non-aqueous electrolyte storage battery, electrolyte storage battery manufacturing, secondary battery and other directions, can solve the requirement that cannot meet the requirements of commercial aluminum-ion battery electrode material, copper-selenium compound aluminum ion has not yet been reported Difficulty in choosing batteries and electrolytes to achieve the effects of abundant reserves, high cycle reversibility and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The aluminum-ion battery positive electrode material of the present embodiment is obtained by the following method: take 2.0g copper nitrate and 0.5g high-purity selenium powder (99.999%), dissolve in deionized water, ethylene glycol, and hydrazine hydrate with a volume ratio of 7:2 : 1 solvent, mix well, transfer the solution to a 100ml hydrothermal kettle, and react at 100°C for 12h. Then wash it several times with ethanol and hot water, and finally dry it in a vacuum oven at 60°C for 12 hours to obtain Cu 2-x Se powder. Will Cu 2-x Se powder, conductive agent acetylene black, and binder polyvinylidene fluoride (PVDF) are mixed and ground according to the mass ratio of 7:2:1, mixed with N-methylpyrrolidone (NMP), fully stirred evenly, and coated on A stainless steel mesh (thickness 0.01 mm) of a suitable size was dried overnight in a vacuum oven at 80° C. to make a positive electrode sheet. The high-purity aluminum sheet is polished with fine sandpaper on both side...
Embodiment 2
[0031] The positive electrode material of the aluminum ion battery of the present embodiment is obtained by the following method: take 2.0g copper chloride and 0.5g high-purity selenium powder (99.999%), dissolve in deionized water, ethylene glycol, and hydrazine hydrate with a volume ratio of 7: 2:1 solvent, mix well, transfer the solution to a 100ml hydrothermal kettle, and react at 100°C for 12h. Then wash with ethanol and hot water for several times, and finally dry in a vacuum oven at 60°C for 12 hours to obtain CuSe powder. Mix and grind CuSe powder, conductive agent acetylene black, and binder polyvinylidene fluoride (PVDF) according to the mass ratio of 7:2:1, mix with N-methylpyrrolidone (NMP), stir well, and coat Put it on a stainless steel net (thickness 0.01mm) of suitable size, and dry it in a vacuum oven at 80° C. overnight to make a positive electrode sheet. The high-purity aluminum sheet is polished with fine sandpaper on both sides, soaked in ethanol for 1:2h...
Embodiment 3
[0033] The aluminum ion battery positive electrode material of the present embodiment is obtained by the following method: take 2.0g copper sulfate and 0.5g high-purity selenium powder (99.999%), dissolve in deionized water, ethylene glycol, and hydrazine hydrate with a volume ratio of 7:2 : 1 solvent, mix well, transfer the solution to a 100ml hydrothermal kettle, and react at 100°C for 12h. Then wash with ethanol and hot water for several times, and finally dry in a vacuum oven at 60°C for 12 hours to obtain CuSe powder. Mix and grind CuSe powder, conductive agent acetylene black, and binder polyvinylidene fluoride (PVDF) according to the mass ratio of 7:2:1, mix with N-methylpyrrolidone (NMP), stir well, and coat Put it on a stainless steel net (thickness 0.01mm) of suitable size, and dry it in a vacuum oven at 80° C. overnight to make a positive electrode sheet. Both sides of the high-purity aluminum sheet are polished with fine sandpaper, soaked in ethanol for 1 to 2 hou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com