Preparation method of 2,3,5,6-tetra-substituted symmetric pyridine
A technology of tetrasubstituted and pyridine, which is applied in the field of preparation of 2,3,5,6-tetrasubstituted symmetrical pyridine, can solve the problems of unavailable raw materials, narrow range of substrates, not economical and green, etc. Effects with a wide range and high compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
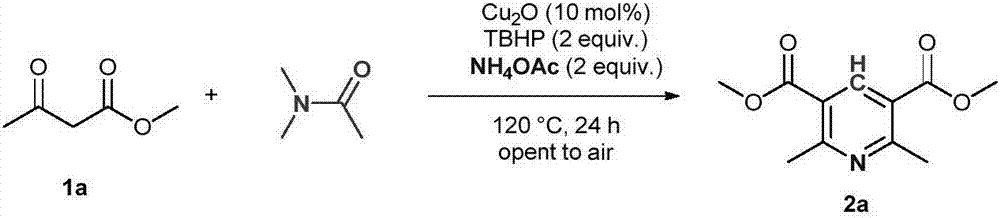
[0014]
[0015] Add 1a (4mmol, 464mg), cuprous oxide (0.4mmol, 57mg), tert-butyl hydroperoxide (8mmol, 70% aqueous solution, 1.2mL), ammonium acetate (8mmol, 616mg) and DMA (10mL) into the reaction flask , And then heated and reacted at 120°C for 24 hours. After the reaction is completed, it is quenched with saturated sodium sulfite solution, and then extracted with ethyl acetate. The organic phases are combined and dried with anhydrous sodium sulfate. After concentration, the product 2a can be obtained by column chromatography with a mixed solvent of petroleum ether and ethyl acetate. The yield was 82%. White solid, Mp: 100-101℃; 1 H NMR(600MHz, CDCl 3 ): δ8.71(s,1H), 3.93(s,6H), 2.86(s,6H); 13 C NMR(150MHz, CDCl 3 ): δ166.2, 162.6, 141.0, 122.6, 52.3, 24.9.
Embodiment 2
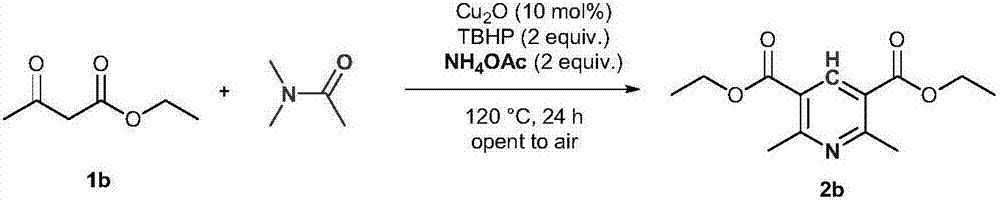
[0017]
[0018] Add 1b (4mmol, 520mg), cuprous oxide (0.4mmol, 57mg), tert-butyl hydroperoxide (8mmol, 70% aqueous solution, 1.2mL), ammonium acetate (8mmol, 616mg) and DMA (10mL) into the reaction flask , And then heated at 120°C for 24 hours. After the reaction is completed, it is quenched with saturated sodium sulfite solution, and then extracted with ethyl acetate. The organic phases are combined and dried with anhydrous sodium sulfate. After concentration, the product 2b is obtained by column chromatography with petroleum ether and ethyl acetate mixed solvent. The yield was 79%. Light yellow solid, Mp: 67-68°C; 1 H NMR(600MHz, CDCl 3 ): δ8.68(s,1H), 4.40(q,J=7.2Hz,4H), 1.42(t,J=7.2Hz,6H); 13 C NMR(150MHz, CDCl 3 ): δ165.9, 162.2, 140.9, 123.0, 61.4, 24.9, 14.2.
Embodiment 3
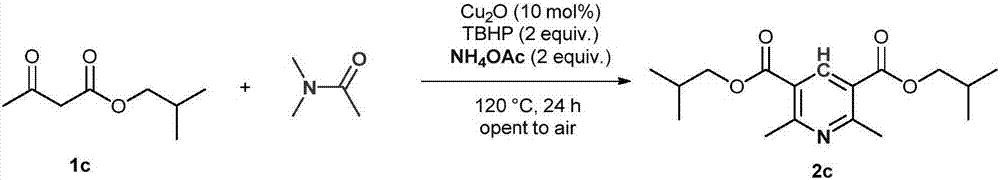
[0020]
[0021] Add 1c (4mmol, 632mg), cuprous oxide (0.4mmol, 57mg), tert-butyl hydroperoxide (8mmol, 70% aqueous solution, 1.2mL), ammonium acetate (8mmol, 616mg) and DMA (10mL) into the reaction flask , And then heated at 120°C for 24 hours. After the reaction is completed, it is quenched with saturated sodium sulfite solution, and then extracted with ethyl acetate. The organic phases are combined and dried with anhydrous sodium sulfate. After concentration, column chromatography is performed with a mixed solvent of petroleum ether and ethyl acetate to obtain product 2c. The yield was 72%. Yellow liquid 1 H NMR(600MHz, CDCl 3 ): δ8.74(s,1H), 4.12(d,J=6.0Hz,4H), 2.87(s,6H), 2.10(m,J=6.6Hz,2H), 1.04(d,J=7.2Hz ,12H); 13 C NMR(150MHz, CDCl 3 ):δ165.8,162.3,141.0,122.9,71.4,27.7,25.0,19.2; HRMS(ESI):calcd for C 17 H 26 NO 4 [M+H] + 308.1856,found308.1861.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com