Keratin biomacromolecular nitric oxide donor and synthesis and application thereof
A biomacromolecule and nitric oxide technology, applied in the field of keratin biomacromolecule nitric oxide donor and its synthesis, can solve the problems of limiting the effectiveness and persistence of biological effects, having biological toxicity, short half-life and the like, Achieve the effect of promoting the growth of vascular endothelial cells, high molecular weight and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
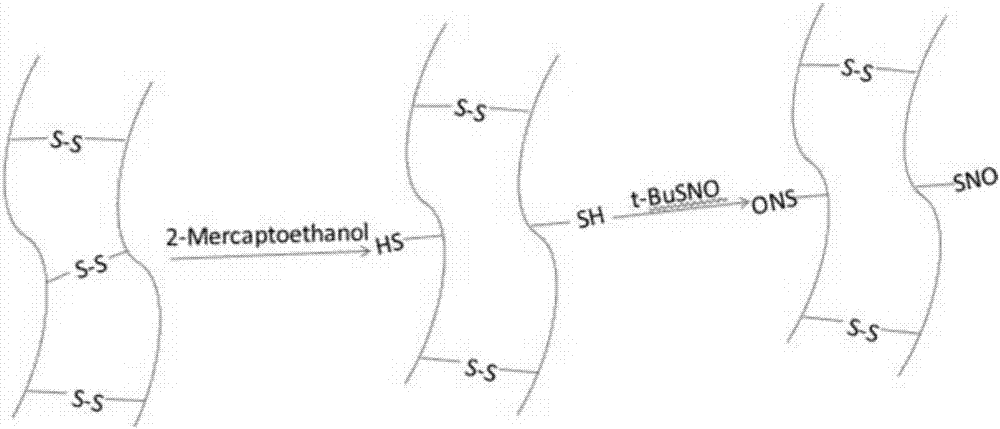
[0028] Embodiment 1: Keratin biomacromolecule nitric oxide donor and its synthesis (oil phase method)
[0029] Take 0.1 g of laboratory-extracted reduced keratin (KSH, mercaptokeratin) and disperse it with 5 mL of ether in a brown bottle, add 1 mL of ether solution of tert-butyl nitrite, keep it in the dark and avoid light, and stir for 24 hours at room temperature under a nitrogen atmosphere. Rotary evaporation at room temperature removes unreacted tert-butyl nitrite, by-products tert-butanol and ether to obtain S-nitrosylated keratin (KSNO), which is a NO donor keratin.
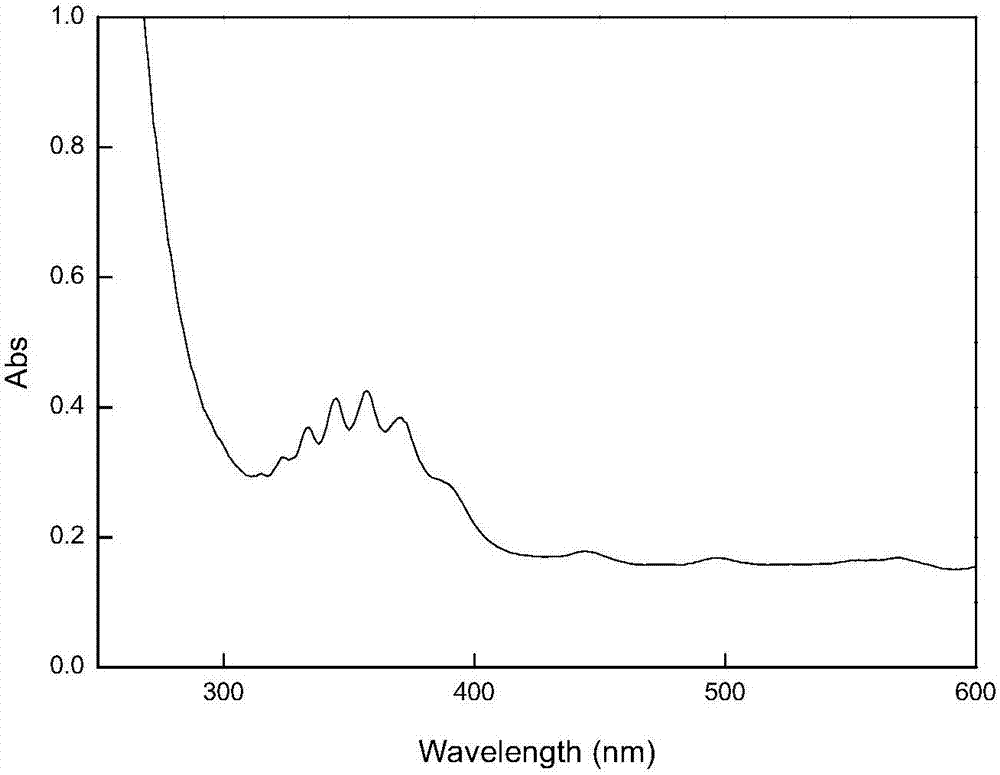
[0030] The above-prepared S-nitrosylated keratin (KSNO) was made into a solution with a certain concentration, and the ultraviolet absorption spectrum was measured in the wavelength range of 200-600nm, such as image 3 shown. The absorption peak at 334nm is the n on the S-NO bond on the modified keratin 0 -π* electron transition absorption (V.B. Damodaran, L.W. Place, M.J. Kipper, M.M. Reynolds.
[0031]...
Embodiment 2
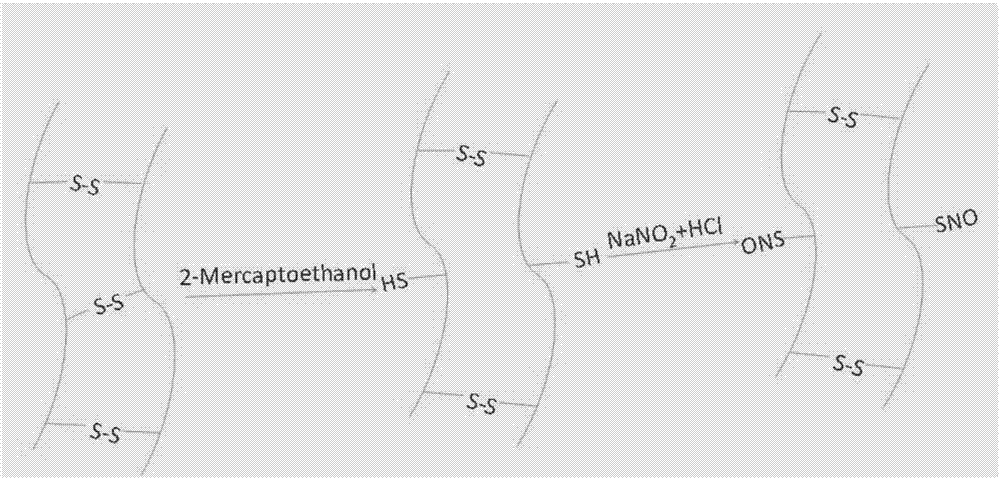
[0033] Embodiment 2: Keratin biomacromolecule nitric oxide donor and its synthesis (aqueous phase method)
[0034] Put 0.1 g of the reduced keratin sample into 2 mL, 50 mg / mL sodium nitrite aqueous solution and stir to dissolve. Under ice bath, slowly add 5mL of 5M hydrochloric acid dropwise. Avoid light and stir for 2h. The excess small molecules were removed by dialysis, and the SNO terminal keratin was obtained by freeze-drying. Samples were stored at -20°C in the dark.
Embodiment 3
[0035] Embodiment 3: Stability experiment of keratin biomacromolecule nitric oxide donor
[0036] Take 20 mg of the keratin biomacromolecule nitric oxide donor KSNO prepared in Example 1 or 2, dissolve it in 5 ml of phosphate buffer, and dialyze the solution at 37° C. with 35 ml of phosphate buffer containing 0.1 mmol EDTA. Set the time to take samples, and use the NO kit to measure the content of NO. The results are as follows: Figure 5 shown. Depend on Figure 5 It can be seen that the keratin biomacromolecule nitric oxide donor has good stability in phosphate buffer solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com