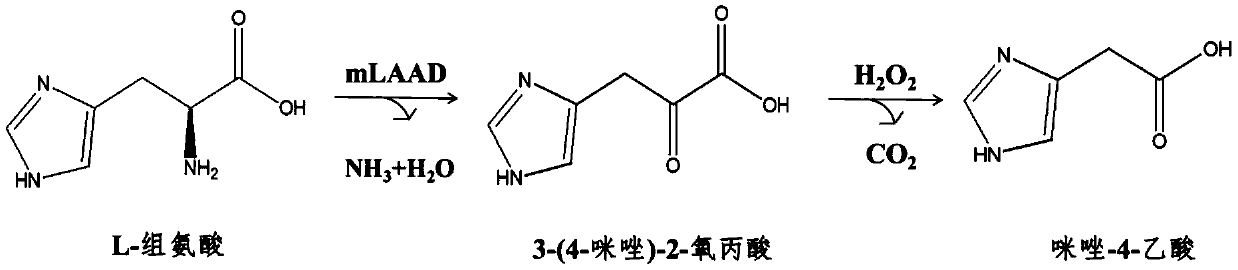
A kind of biosynthesis method of imidazole-4-acetic acid
A biosynthesis and imidazole technology, applied in the field of biochemical engineering, can solve the problems of harsh reaction conditions, large environmental pollution, high cost of raw materials, etc., and achieve the effect of low equipment requirements, high substrate conversion rate, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 Construction of membrane-linked L-amino acid deaminase engineering bacteria
[0025] According to the gene sequence of Proteus vulgaris IAM12003 membrane-linked L-amino acid deaminase (its sequence accession number is BAA90864), the whole gene synthesis was carried out, and the synthetic gene (LAAD) was linked into pET20 ( In b)+, Escherichia coli DH5α strain was transformed, and positive clones were screened to obtain recombinant plasmid pET20b-laad. In addition, the LAAD gene was linked into pET28a(+) through NcoI and BamHI restriction sites, transformed into Escherichia coli DH5α strain, and positive clones were screened to obtain the recombinant plasmid pET28a-laad.
[0026] The two recombinant plasmids were transformed into Rosstta(DE3), wherein the LAAD expressed by Rosstta(DE3) / pET20b-laad contained the PelB signal peptide, and was expressed in the periplasm of the cell using the conserved secretory pathway, while Rosstta(DE3) / pET20b-laad pET28a-laa...
Embodiment 2
[0027] Example 2 Synthesis of imidazole-4-acetic acid by membrane-linked L-amino acid deaminase engineering bacteria
[0028] Specific steps are as follows:
[0029] (1) Pick engineering bacteria (E.coli Rosstta(DE3) / pET28a-laad) from the activated LB solid medium, insert the monoclonal bacteria into -1 ) in LB liquid medium, 37°C, 200r / min shaking culture overnight, to obtain seed liquid.
[0030] (2) Inoculate the seed solution with a volume fraction of 2% inoculum into a seed containing kanamycin (50 μg·mL -1 ) of LB medium, cultured at 37°C, 200r / min until OD 600 At 0.6, add IPTG to make the final concentration of IPTG 0.5 μM, induce culture at 30° C., 150 r / min for 6 h.
[0031] (3) Collect the engineering bacteria cells by centrifugation, discard the supernatant, and wash the cells with 0.2mM phosphate buffer (pH 7.5). Add the cleaned engineered bacteria into a phosphate buffer (0.2mM, pH 7.5) containing 50mM L-histidine, and the density of the bacteria in the solut...
Embodiment 3
[0033] Example 3 Utilize membrane-linked L-amino acid deaminase engineering bacteria to synthesize imidazole-4-acetic acid
[0034]Adjust the L-histidine concentration of the reaction solution in step (3) of Example 2 to 100 mM, and keep other conditions unchanged. Cultivate for 37 hours at 37° C. and 200 r / min to obtain a solution containing 3-(4-imidazole)-2-oxopropionic acid. To this solution was added 1% H 2 o 2 , and reacted for 10 minutes to obtain an imidazole-4-acetic acid solution with a content of 35.6 mM, and the conversion rate of histidine to imidazole-4-acetic acid reached 37%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com