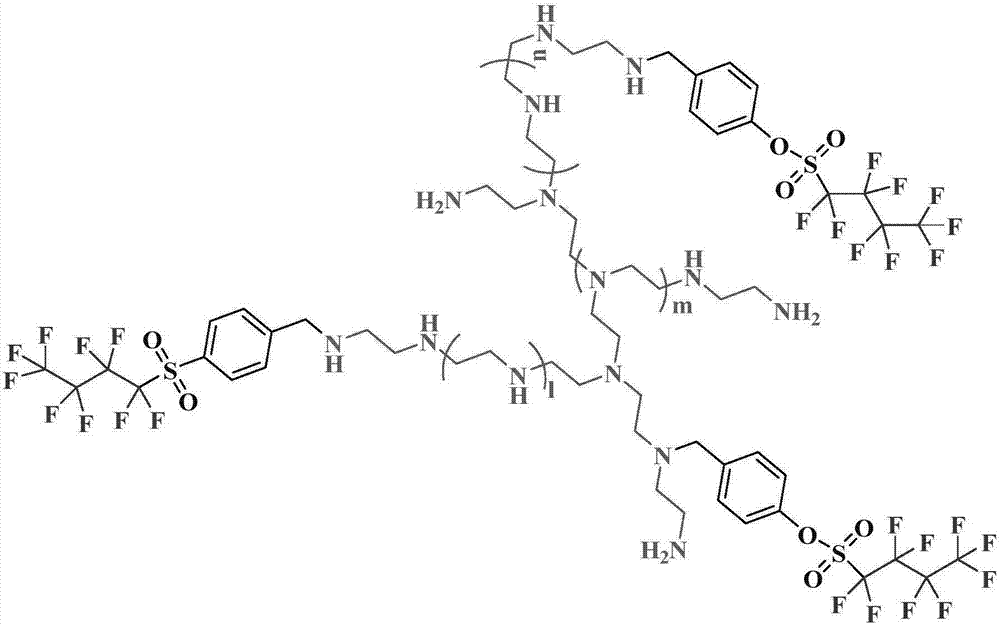
Perfluorobutyl modified polyethyleneimine macromolecular fluorine-containing surfactant as well as preparation and application thereof in slow release of pesticides
A technology of polyethyleneimine and surfactants, which is applied in the fields of application, chemicals for biological control, biocides, etc. It can solve the problems of poor selectivity, low water solubility, and short duration, etc., and achieve excellent surface Activity, optimized micelle morphology, excellent use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1 intermediate (M1)
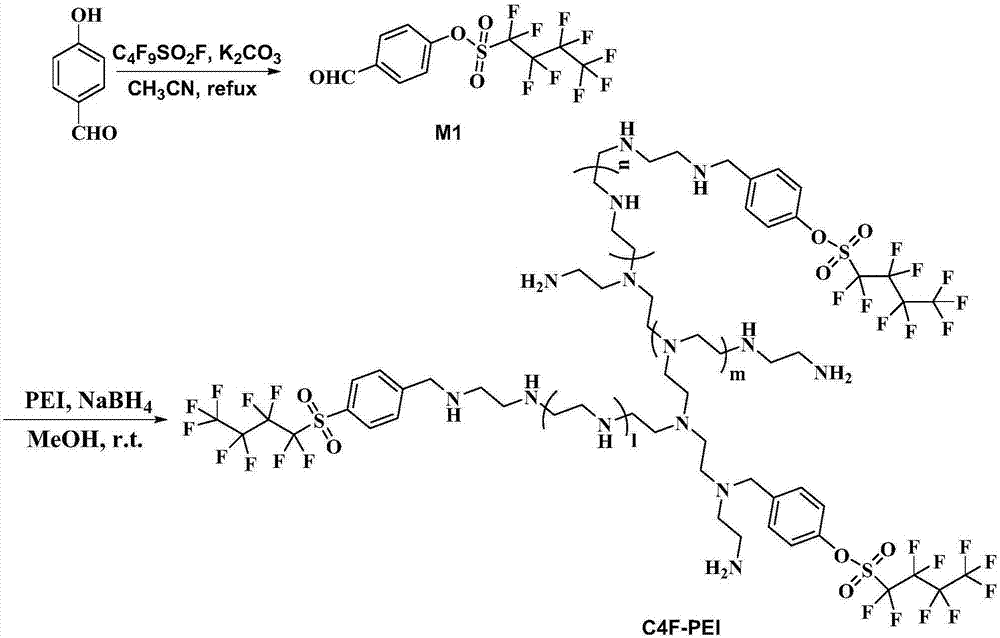
[0045] Synthetic route such as figure 2 As shown, add 12.2g (0.1mol) p-hydroxybenzaldehyde in a dry 250mL flask, dissolve it with 150mL acetonitrile, add 27.6g (0.2mol) of acid-binding agent potassium carbonate under stirring, reflux for half an hour, and then slowly dropwise add The fluorine precursor raw material was 36.2 g (0.12 mol) of perfluorobutylsulfonyl fluoride, and the end point of the reaction was monitored by TLC. Add 100mL ethyl acetate to the reaction solution, wash 3 times with saturated sodium chloride solution, dry the organic layer with anhydrous sodium sulfate and crystallize at low temperature, filter to obtain the white solid intermediate perfluorobutylsulfonyloxybenzaldehyde (M1 ), 39.2g, the yield is 97%.
[0046]
[0047] 1 H NMR (600MHz, DMSO): δ10.08 (s, 1H, -CHO), 8.13 (d, J = 8.0Hz, 2H, phH), 7.75 (d, J = 8.0Hz, 2H, phH);
[0048] 19 F NMR(376MHz,DMSO):δ-82.01(3F),-112.34(2F),-121....
Embodiment 2
[0050] Example 2 Preparation of perfluorobutyl modified PEI macromolecular fluorosurfactant
[0051] Synthetic route such as figure 2 As shown, in a dry 250mL flask, add 8g (0.02mol, 5% of the total moles of primary and secondary amines in the following PEI) perfluorobutylsulfonyloxybenzaldehyde (M1), 100mL of anhydrous methanol, Then add 22.6g PEI (M.W.1800), stir at room temperature for 2h, then add sodium borohydride 0.75g (0.02mol) in batches, continue to react for 10min, and monitor the reaction end point by TLC. After the reaction is completed, add acetone to dissolve again, white solid impurities are precipitated, and the filtrate is obtained by filtration, and the filtrate is repeatedly precipitated with n-hexane to obtain a yellow oily product, which is labeled as perfluorobutyl modified PEI macromolecular fluorosurfactant C4F -PEI-B, yield 92%. pass 1 The ratio of the proton peak integral area on the intermediate benzene ring in H NMR to the proton peak and sub-a...
Embodiment 3
[0053] Example 3 Test of surface tension and critical micelle concentration (CMC) of perfluorobutyl modified PEI macromolecular fluorosurfactant
[0054] Take a certain amount of the above C4F-PEI-B, and use distilled water to make solutions with the following concentration gradients (unit: mg / mL): 0.1, 0.5, 0.7, 0.9, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0 , 8.0, 9.0, 10.0, 11.0, 12.0, use the OCA20 contact angle measuring instrument to measure the surface tension at 25°C (hanging drop method), the minimum surface tension is 22.6mN / m, and the critical micelle concentration (CMC) is 3.0 mg / mL, the test results are shown in the figure Figure 4 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Minimum surface tension | aaaaa | aaaaa |
| Critical micelle concentration | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com