Hexagonal boron-nitride material, preparation method and application of hexagonal boron-nitride material
A technology of hexagonal boron nitride and boron nitride, which is applied in the field of hexagonal porous boron nitride material and its preparation, can solve the problem of uneven structure of boron nitride pore, uncontrollable morphology of boron nitride, controllable adjustment of precursor, etc. problem, to achieve the effect of improving the adsorption effect, improving the adsorption, and increasing the specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] This embodiment provides a method for preparing the hexagonal boron nitride material provided by the application, and the specific method is as follows:
[0089] (1) Dissolve 15.3g of boric acid and melamine in 250ml of water at a molar ratio of 4:1, adjust the pH of the solution to 10 with sodium hydroxide, heat to 90°C and fully stir for 4h, then cool and crystallize at 25°C for 12h Filtrate, wash the separated solid, and dry in a drying oven at 60°C to obtain precursor C 3 N 6 h 6 · 2H 3 BO 3 ;
[0090] (2) Under pure ammonia atmosphere, the precursor C obtained in step (1) 3 N 6 h 6 2H 3 BO 3 First heat up to 600°C for 2 hours at a rate of 3°C / min, then heat up to 700°C for 3 hours at a rate of 4°C / min, and obtain the hexagonal boron nitride material after reduction and nitriding.
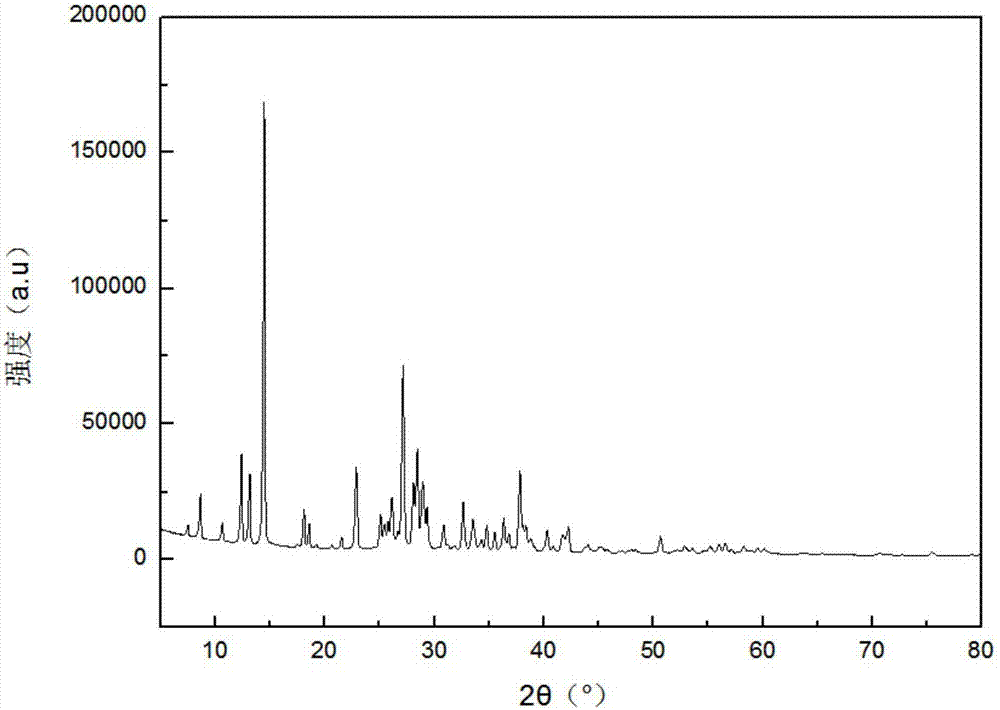
[0091] figure 1 It is the X-ray diffraction (XRD) pattern of the precursor prepared by step (1) of the present embodiment, precursor C 3 N 6 h 6 2H 3 BO 3 It is not a sim...
Embodiment 2
[0098] This embodiment provides a method for preparing the hexagonal boron nitride material provided by the application, and the specific method is as follows:
[0099] (1) Dissolve 6.3 g of boric acid and melamine in 250 ml of water at a molar ratio of 2:1, adjust the pH of the solution to 5 with hydrochloric acid, and take the total mass of boric acid, melamine and optional surfactants as 100%, add 15wt% surfactant DTAC, heated to 90°C and fully stirred for 5h until the solution became transparent, cooled and crystallized at 25°C for 20h, filtered, washed the separated solid, and dried in a drying oven at 60°C to obtain precursor C 3 N 6 h 6 2H 3 BO 3 ;
[0100] (2) Under the atmosphere of pure ammonia and nitrogen 1:1, the precursor C obtained in step (1) 3 N 6 h 6 · 2H 3 BO 3 First heat up to 600°C for 3 hours at a rate of 3°C / min, then heat up to 800°C for 3 hours at a rate of 3°C / min, and obtain the hexagonal boron nitride material after reduction and nitriding....
Embodiment 3
[0104] This embodiment provides a method for preparing the hexagonal boron nitride material provided by the application, and the specific method is as follows:
[0105] (1) Dissolve 12.6g of boric acid and melamine in 2:1 molar ratio in total in 500ml of water, adjust the pH of the solution to 8 with sodium hydroxide, heat to 88°C and fully stir for 6h, cool and crystallize at 25°C for 18h Filtrate, wash the separated solid, and dry in a drying oven at 80°C to obtain precursor C 3 N 6 h 6 · 2H 3 BO 3 ;
[0106] (2) Under the atmosphere of pure ammonia and nitrogen 2:1, the precursor C obtained in step (1) 3 N 6 h 6 · 2H 3 BO 3 First heat up to 500°C for 2 hours at a rate of 5°C / min, then heat up to 1000°C for 3 hours at a rate of 4°C / min, and obtain the hexagonal boron nitride material after reduction and nitriding.
[0107] Figure 7 It is the SEM photo of the hexagonal boron nitride material prepared in this example. It can be seen that the hexagonal boron nitride...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Sheet thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com