A kind of topicastat microsphere preparation and preparation method thereof
A technology of topinostat and preparations, which is applied in the field of topinostat microsphere preparations and its preparation, can solve problems such as inconvenient administration and limitations in clinical application, achieve stable quality, increase drug delivery routes, and improve compliance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
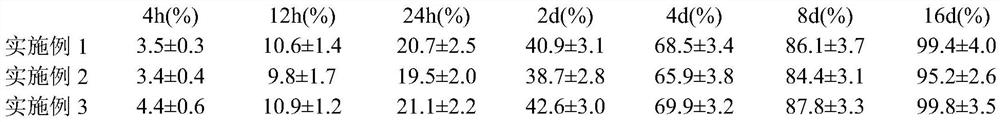
Embodiment 1
[0023]Weigh 10g of topipristat and dissolve it in 20mL of dichloromethane as the dispersed phase; weigh 40g of polylactic acid hydroxylactic acid and 3g of Span 80 and dissolve them in 100mL of ethanol aqueous solution as the continuous phase, and slowly add the dispersed phase dropwise to the organic phase Emulsify the emulsion at 30°C and 2000r / min for 20 minutes under stirring conditions. Pour the resulting emulsion into 400ml of acetone to solidify, wash the sediment with acetone until there are no oil droplets; prepare a 5% mannitol solution and add 100mL to the sediment In the medium, stir evenly and freeze-dry in vacuum. The vacuum freeze-drying process includes pre-freezing at -40°C for 2 hours, slowly raising the temperature to -5°C and vacuum drying for 10 hours, slowly raising the temperature to 10°C and vacuum drying for 8 hours, and continuing to raise the temperature to 30°C for 1 hour. The encapsulation rate of topipristat in the microspheres is 88.2±4.1%, and the par...
Embodiment 2
[0025]Weigh 20g of Topipristat and dissolve it in 20mL of chloroform as the dispersed phase; Weigh 80g of chitosan and 2g of sodium lauryl sulfate, dissolve into 200mL of ethanol aqueous solution as the continuous phase, slowly add the dispersed phase to the organic In the phase, ultrasonic emulsification for 30 minutes to form an oil-in-water emulsion; the organic solvent is removed by rotary evaporation at 40°C, 100 mL of 3% glucose solution is added, and the product is obtained by vacuum freeze drying. The vacuum freeze-drying process includes pre-freezing at -30°C for 4 hours, slowly raising the temperature to 3°C for 12 hours, slowly raising the temperature to 15°C for 6 hours, and continuing to raise the temperature to 32°C for 2 hours. The encapsulation rate of topistat in the microspheres is 92.5±3.7%, and the particle size is 0.54±0.04μm.
Embodiment 3
[0027]Weigh 15 g of topicastat and dissolve it in 20 mL of dichloromethane; weigh 100 g of polylactic acid and dissolve it in 200 mL of ethanol aqueous solution, mix the two solutions and spray-dry to prepare the topicinostat microsphere preparation. The spray drying conditions are: the atomization pressure is 15MPa, the material liquid temperature is 50°C, the hot air temperature in the spray drying tower is 110°C, and the feed pump speed is 5r / min. The encapsulation rate of topistat in the microspheres is 80.8±2.9%, and the particle size is 0.96±0.07μm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com