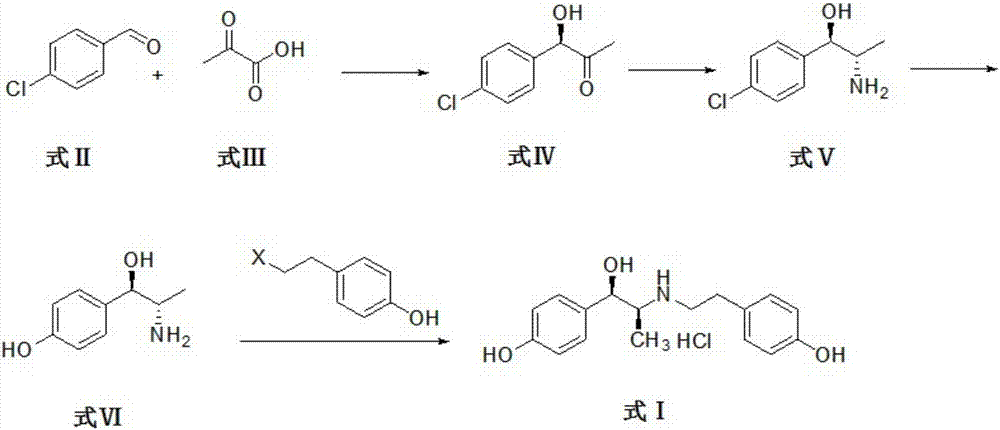
Method for synthesizing ritodrine hydrochloride
A technique for synthesizing ritodrine hydrochloride, which is applied in the fields of chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxyl compounds, etc., can solve problems such as complicated process and achirality of ritodrine, and achieve simple process flow, The effect of cost reduction and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The whole cell of genetically engineered bacteria derived from Zymomonas mobilis phenylacetone synthase is prepared by selecting the gene sequence of phenylacetone synthase derived from Zymomonas mobilis and artificially designing it. The designed gene sequence is as SEQ ID NO in the sequence table : The nucleotide sequence shown in 1 is shown in the sequence; the sequence is synthesized by the whole gene, cloned into the Nde I and Xho I restriction sites of the expression vector pET28a, and transformed into competent cells of the host bacteria E.coli BL21(DE3); After picking the positive transformants and sequencing for identification, a recombinant expression vector is obtained; the recombinant expression vector is transferred into E.coli BL21(DE3) strain to obtain a recombinant phenylacetone synthase genetically engineered strain that can induce expression of recombinant phenylacetone synthase. The recombinant phenylacetone synthase genetically engineered bacteria was ...
Embodiment 2
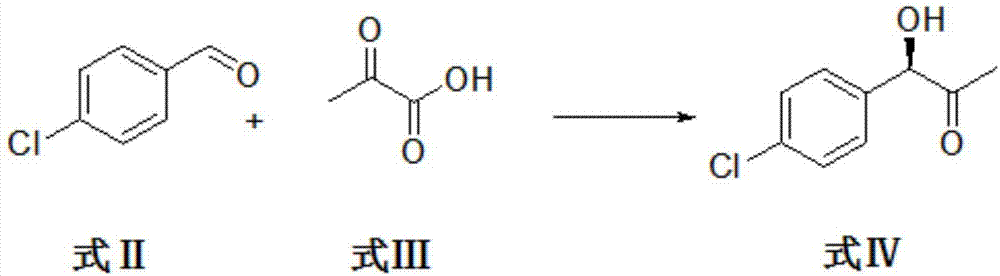
[0036] The reaction system is composed of p-chlorobenzaldehyde compound of formula II and pyruvate compound of formula III as substrates and buffer solution, catalyst and additives are added, reacted and purified to obtain compound of formula IV (R)-1-(4-chlorophenyl) -1-Hydroxypropan-2-one.
[0037] The specific reaction process is as follows: the reaction is carried out in a 1L shake flask, the reaction system is controlled to 300mL, and the compound of formula II p-chlorobenzaldehyde (29.51g, 0.21mol) and the compound of formula III pyruvic acid (24.64g, 0.28mol) are used as substrates. , Using citric acid-sodium citrate buffer solution as solvent, using genetically engineered bacteria whole cells derived from Zymomonas mobilis phenylacetone synthase and coenzyme thiamine pyrophosphate as catalyst. Control the concentration of whole cells of genetically engineered bacteria derived from Zymomonas mobilis phenylacetone synthase to 100g / L, and control the concentration of coenzym...
Embodiment 3
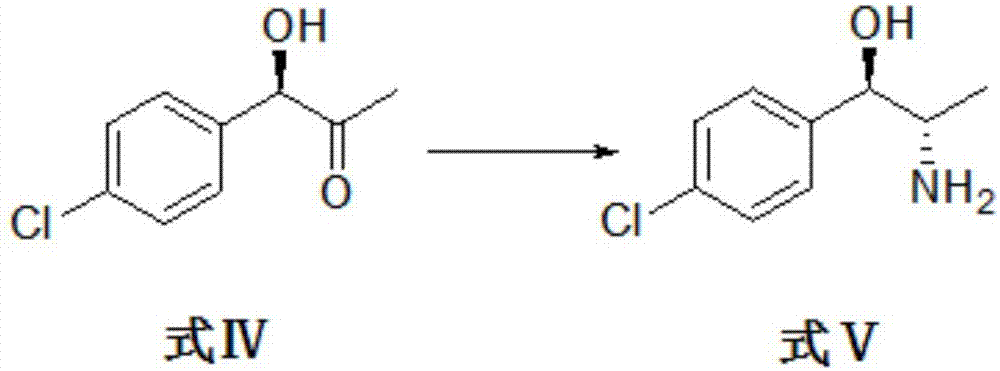
[0041] Take formula IV compound (R)-1-(4-chlorophenyl)-1-hydroxypropan-2-one and ammonium formate as substrates, form a reaction system with buffer solution, add catalyst, react, and purify to obtain formula V The compound (1R, 2S)-2-amino-1-(4-chlorophenyl)-1-propanol.
[0042] The reaction was carried out in a 1L shake flask, the reaction system was controlled to 300 mL, and the compound of formula IV (R)-1-(4-chlorophenyl)-1-hydroxypropan-2-one (35.06g, 0.19mol) and ammonium formate (23.94g, 0.38mol) as the substrate, the phosphate buffer solution as the solvent, the genetically engineered bacterial fragmentation enzyme solution of leucine dehydrogenase derived from Saccharomyces cerevisiae, the gene of formate dehydrogenase derived from Candida boidinii Engineering bacteria cell crushing enzyme solution and coenzyme NAD+ are catalysts. Control the concentration of the disrupted enzyme solution of genetically engineered bacteria derived from Saccharomyces cerevisiae to 10g / L,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com