Bio-preparation method for pseudoephedrine
A technique for pseudoephedrine and biological preparation, applied in the field of biological preparation of pseudoephedrine, can solve problems such as cost and environmental protection, unfavorable actual production, high cost, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The genetic engineering bacteria derived from the phenylacetone synthase of Acetobacter pasteurii, the specific preparation method is:
[0061] (1) construct the expression vector that contains phenylacetone synthase gene:
[0062] The genomic DNA of the phenylacetone synthase of Acetobacter pasteurii was extracted as a template, and the following nucleotide sequences containing NdeI and XhoI restriction sites were used as primers for PCR amplification:
[0063] Primer 1:5'—GAATT CATATG ACCTATACCGTGGGCATG—3’
[0064] Primer 2:5' - GCC CTCGAG TTACGCCAGGGTGGTT—3’
[0065] The NdeI restriction site and the XhoI restriction site were introduced into the underlined portion of the 5' end of the above primers, respectively.
[0066] The PCR amplification system is: genomic DNA 2uL, primer 1 and primer 2 each 2uL, dNTP 4uL, 10×Taq buffer 5uL, Taq enzyme 1uL, ddH 2 O 34uL;
[0067] The PCR reaction program was as follows: pre-denaturation at 94 °C for 2 min; denaturation ...
Embodiment 2
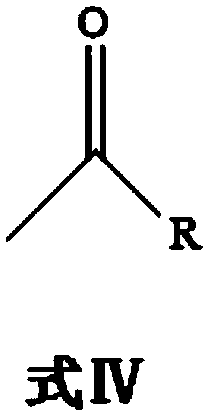
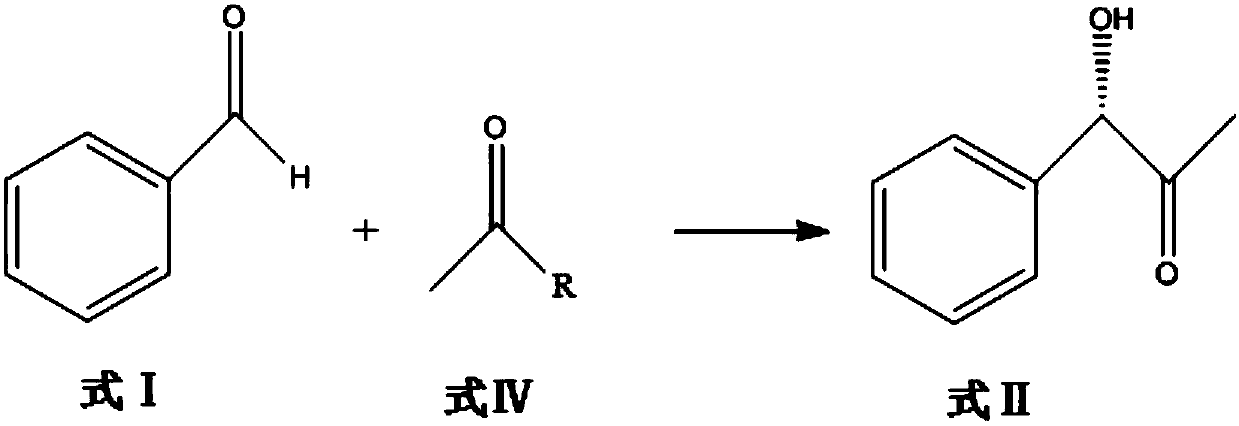
[0091] The compound of formula I, benzaldehyde, and the compound of formula IV are reacted in a buffer solution under the catalysis of biological enzymes, and purified to obtain optically pure compound of formula II (S)-1-phenyl-2-carbonyl-3-propanol;
[0092] The specific reaction process is as follows: the reaction is carried out in a 1L shake flask, the reaction system is controlled to 300mL, and the compound of formula I benzaldehyde (30g, 0.28mol) and the compound of formula IV pyruvic acid (35.2g, 0.4mol) are used as substrates, and lemon is used as the substrate. The acid-sodium citrate buffer solution is used as a solvent, and the whole cell of genetically engineered bacteria derived from the phenylacetone synthase of Acetobacter pasteurii is used as a catalyst, and coenzyme thiamine pyrophosphate is added. The concentration of the whole cell of the genetically engineered bacteria that controls the phenylacetone synthase derived from Acetobacter pasteurii is 70 g / L, and...
Embodiment 3
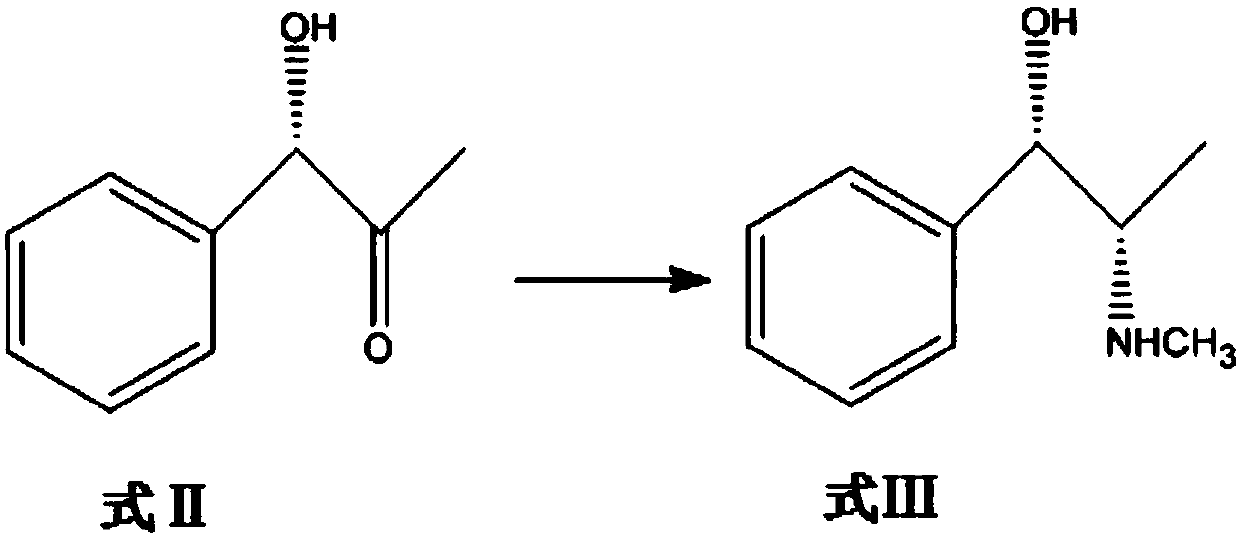
[0096] Optically pure compound of formula II (S)-1-phenyl-2-carbonyl-3-propanol reacts with methylamine in a buffer solution under the catalysis of biological enzymes to generate pseudoephedrine;
[0097] The reaction was carried out in a 1L shake flask, the reaction system was controlled to 300mL, and the compound of formula II (S)-1-phenyl-2-carbonyl-3-propanol (30g, 0.2mol) and methylammonium (15.5g, 0.5mol) were mixed with ) as a substrate, adding glucose (45g, 0.25mol), using phosphate buffer solution as a solvent, using the genetically engineered bacteria whole cell and glucose dehydrogenase derived from the N-methyl amino acid dehydrogenase of Pseudomonas putida ATCC12633 as catalysts , add the coenzyme NAD+. The whole cell concentration of the genetically engineered bacteria controlling N-methyl amino acid dehydrogenase derived from Pseudomonas putida ATCC12633 was 28 g / L, and the concentration of the control coenzyme NAD+ was 0.4 g / L. The pH value of the transformati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com