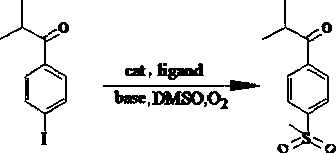Preparation method of Firocoxib intermediate
A technology of firocoxib and intermediates, which is applied in the field of preparation of firocoxib intermediates, can solve the problems of failure to meet environmental requirements, respiratory tract damage, etc., and achieve the effects of increased yield, improved purity, and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Under air or oxygen atmosphere, add 2mL dimethylsulfoxide to the reactor, then add 68.25mg 1-(4-iodophenyl)-2-methylpropan-1-one, 25.03mg acetylacetone, 3.58mg Cuprous oxide, 2.8mg of palladium acetate and 84mg of potassium tert-butoxide were mixed and stirred evenly, the temperature of the system was raised to 100°C, and the reaction was refluxed for 20h. After the reaction was completed, 20mL of deionized water was added to the solution, and then 20mL of ethyl acetate was added to carry out After multiple extractions, the organic phases were collected and combined, then dried by adding anhydrous sodium sulfate, and finally the solvent was removed by vacuum evaporation and chromatographic separation to obtain 2-methyl-1-[4-(methylsulfonyl)phenyl] Propan-1-one, 58% yield.
[0026] Adding a catalyst to the reaction system greatly increases the reaction rate, when the amount of palladium salt catalyst is less than 0.001 equivalent of the amount of halogenated aromatic hyd...
Embodiment 2
[0034] Under air or oxygen atmosphere, add 2mL dimethylsulfoxide to the reactor, then add 68.25mg 1-(4-iodophenyl)-2-methylpropan-1-one, 65.50mg triphenylphosphine, 3.58mg of cuprous oxide, 2.8mg of palladium acetate and 84mg of potassium tert-butoxide, mix and stir evenly, raise the temperature of the system to 100°C, reflux for 20h, add 20mL of deionized water to the solution after the reaction, and then add 20mL of ethyl acetate The ester was extracted several times, after collecting and combining the organic phases, adding anhydrous sodium sulfate for drying, and finally removing the solvent by evaporation under reduced pressure and chromatographic separation to obtain 2-methyl-1-[4-(methylsulfonyl)benzene Base] propan-1-one, the yield was 27%.
Embodiment 3
[0036] Under air or oxygen atmosphere, add 2mL dimethylsulfoxide to the reactor, then add 68.25mg 1-(4-iodophenyl)-2-methylpropan-1-one, 25.03mg acetylacetone, 3.58mg Cuprous oxide, 2.8 mg of palladium acetate and 51 mg of sodium ethoxide, mixed and stirred evenly, the temperature of the system was raised to 100 ° C, and the reaction was refluxed for 20 hours. After the reaction was completed, 20 mL of deionized water was added to the solution, and then 20 mL of ethyl acetate was added for several times. After extraction, the combined organic phases were collected, dried by adding anhydrous sodium sulfate, and finally evaporated under reduced pressure to remove the solvent and chromatographically separated to obtain 2-methyl-1-[4-(methylsulfonyl)phenyl]propane- 1-Kone in 38% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com