12-vitamin freeze-dried preparation for injection, and preparation method thereof
A technology of freeze-dried preparations and vitamins, which is applied in freeze-dried transportation, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. problems, to achieve the effect of increasing drug safety, increasing compliance and safety, and reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
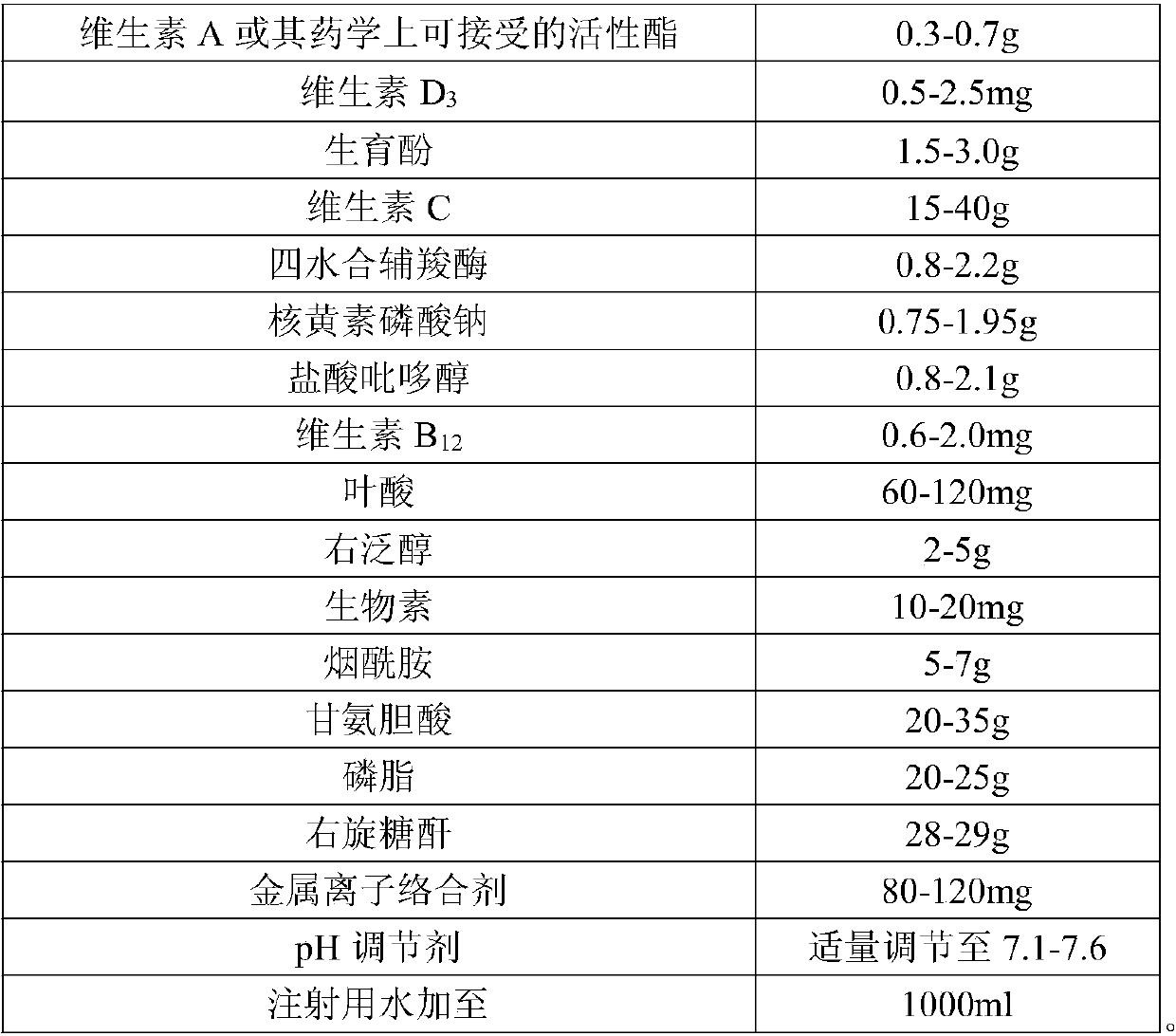
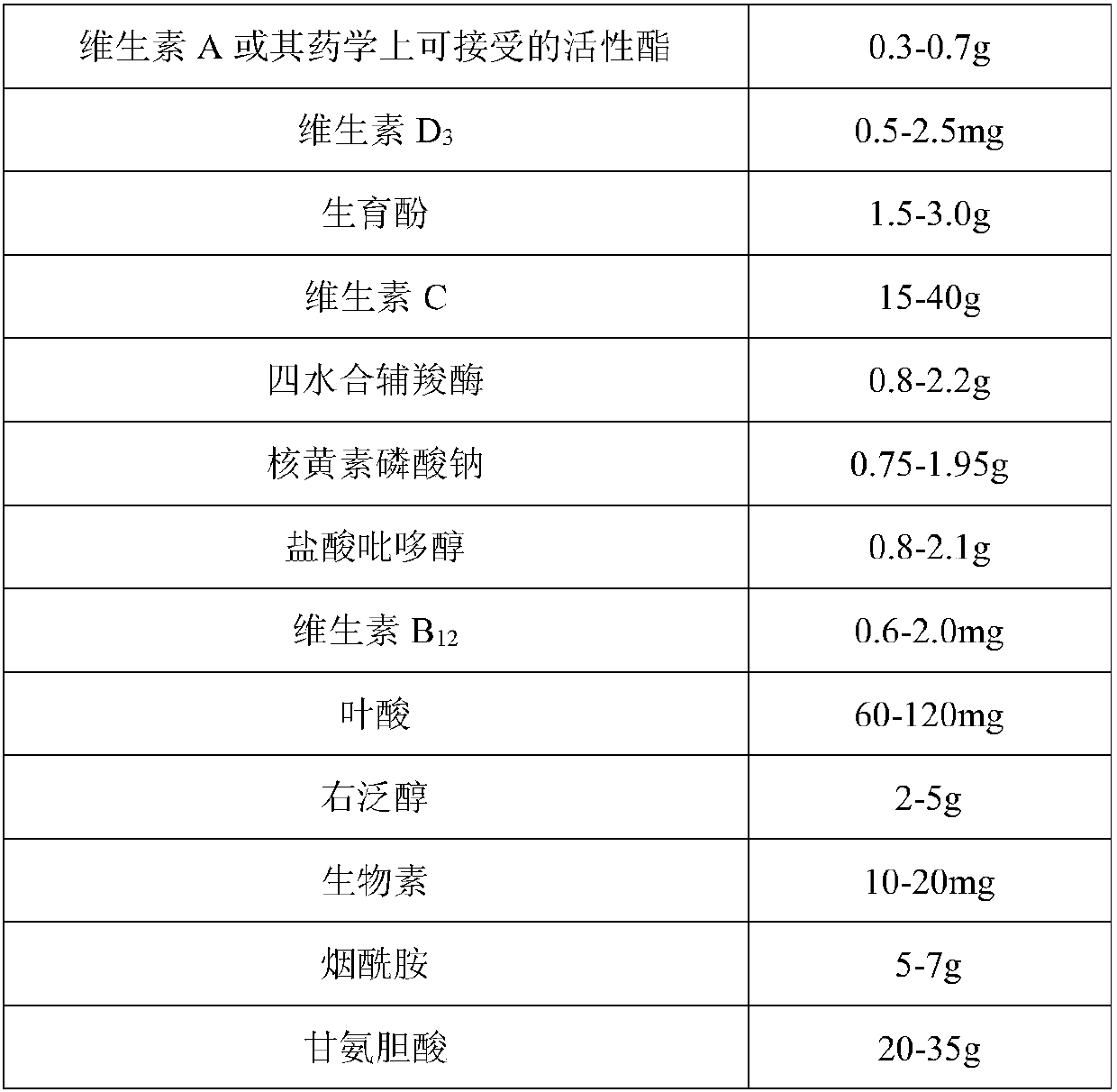
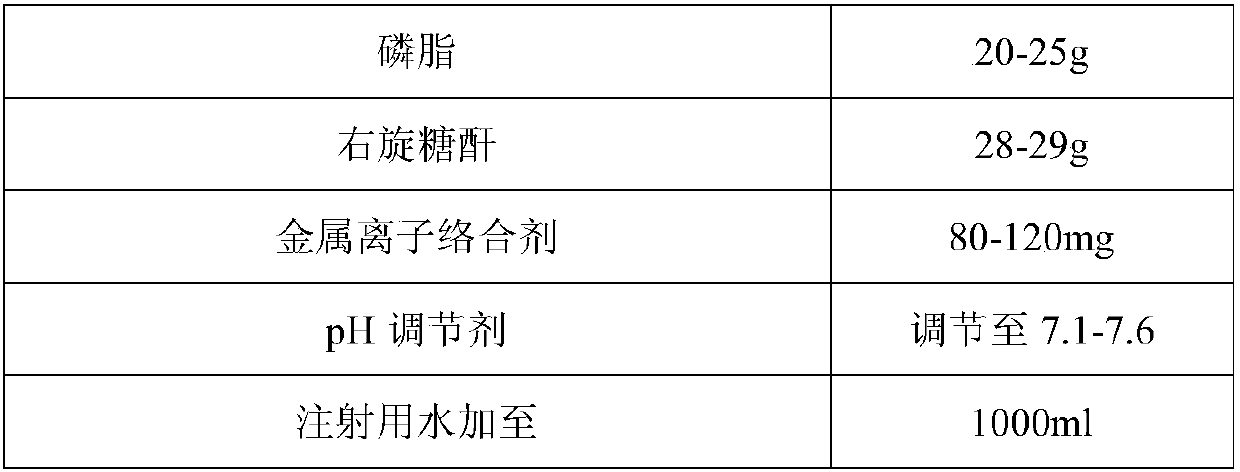
[0033] (1) Preparation of water-soluble components:
[0034] Dissolve the prescribed amount of water-soluble materials (vitamin C, cocarboxylase tetrahydrate, riboflavin sodium phosphate, pyridoxine hydrochloride, dexpanthenol, nicotinamide, dextran, metal ion complexing agent) in an appropriate amount of water for injection one by one , stirring and mixing to obtain a water-soluble medicinal solution A.
[0035] (2) Preparation of fat-soluble ingredients
[0036] Weigh the phospholipids of the prescribed amount, heat to dissolve, add the prescribed amount of glycocholic acid and an appropriate amount of water for injection, stir and dissolve, and after cooling to room temperature, add fat-soluble vitamins (vitamin A or its pharmaceutically acceptable active ester, tocopherol, etc.) one by one. phenol), stirring and dissolving to obtain fat-soluble medicinal solution B.
[0037] (3) mixing:
[0038] ① Mix and stir the obtained water-soluble medicinal liquid A and fat-solubl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com