Preparation method for feed-grade copper citrate
A copper citrate, feed-grade technology, applied in the field of preparation of feed-grade copper citrate, can solve the problems of eye conjunctiva irritation, septal perforation, and elevated body temperature, and achieve the effects of reducing waste liquid discharge, reducing costs, and being easy to handle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
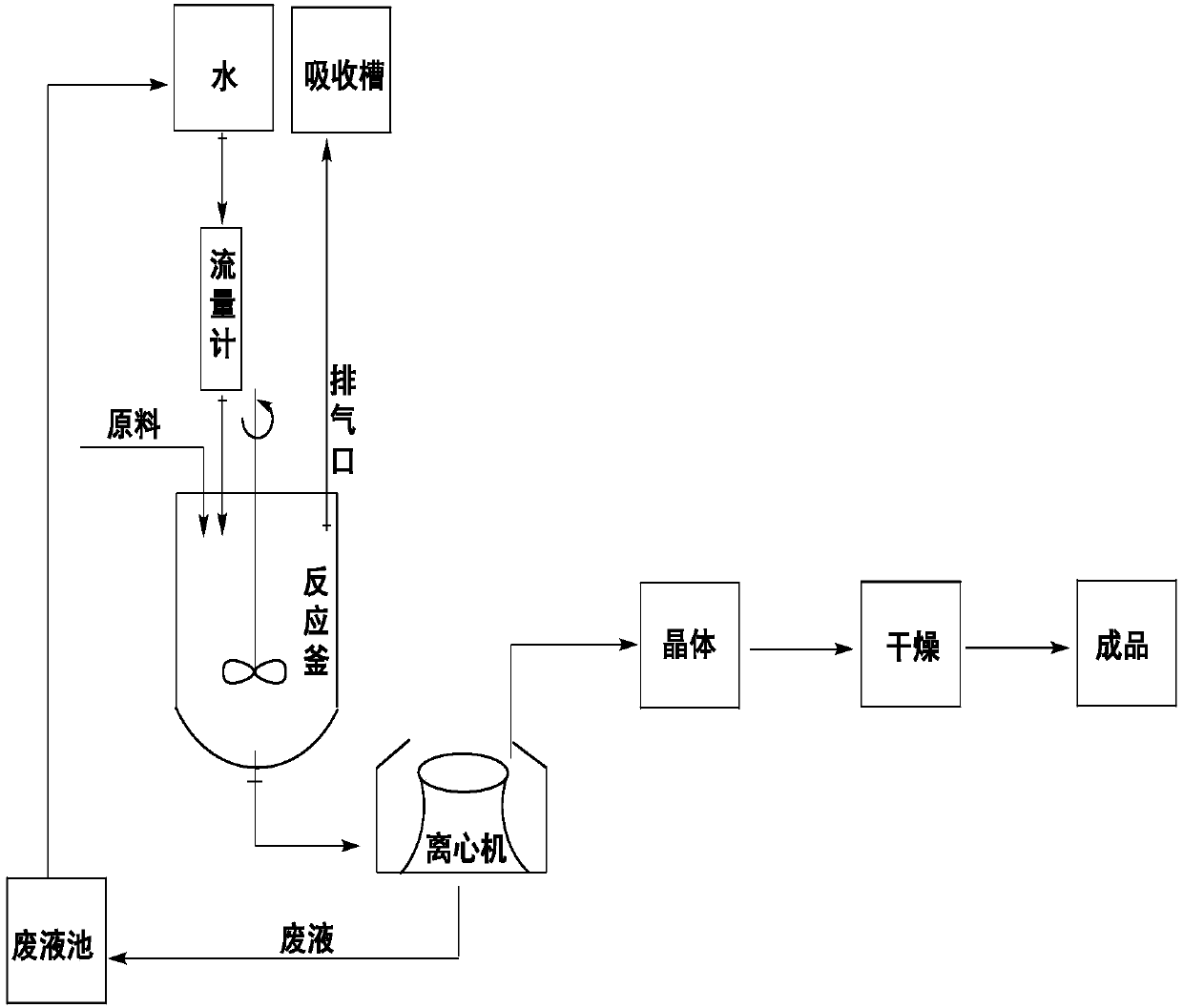
[0022] A preparation method for feed grade copper citrate, comprising the following steps:
[0023] (1) 100kg of citric acid and 400kg of deionized water are metered into the reactor to form a 20% aqueous solution of citric acid and heated to 80°C;
[0024] (2) open agitator, slowly add 100kg basic copper carbonate and carry out neutralization reaction, generate copper citrate, utilize citric acid to control reaction solution pH value to be 4, the CO produced 2 The gas enters the absorption tank filled with NaOH solution through the exhaust port to be absorbed;
[0025] (3) After filtering, concentrating, and cooling to room temperature, the copper citrate generated is put into a centrifuge for dehydration to obtain copper citrate crystals, and the separated waste liquid is discharged into the waste liquid pool for recycling;
[0026] (4) Put the obtained copper citrate crystals into a desiccator and dry at 100° C. for 1 hour to remove part of the crystal water to obtain 150....
Embodiment 2
[0029] A preparation method for feed grade copper citrate, comprising the following steps:
[0030] (1) 100kg of citric acid and 300kg of water (the mixture of deionized water and separation waste liquid) are metered into the reactor to form a 25% aqueous solution of citric acid and heated to 100°C;
[0031] (2) open agitator, slowly add 100kg basic copper carbonate and carry out neutralization reaction, generate copper citrate, control reaction solution pH value to be 6, the CO produced 2 The gas enters the absorption tank filled with NaOH solution through the exhaust port to be absorbed;
[0032] (3) After filtering, concentrating, and cooling to room temperature, the copper citrate generated is put into a centrifuge for dehydration to obtain copper citrate crystals, and the separated waste liquid is discharged into the waste liquid pool for recycling;
[0033] (4) Put the obtained copper citrate crystals into a desiccator and dry at 120° C. for 2 hours to remove part of th...
Embodiment 3
[0036] A preparation method for feed grade copper citrate, comprising the following steps:
[0037] (1) 100kg of citric acid and 376kg of water (the mixed solution of deionized water and separation waste liquid) are metered into the reactor to form a 21% aqueous solution of citric acid and heated to 90°C;
[0038] (2) open agitator, slowly add 90kg basic copper carbonate and carry out neutralization reaction, generate copper citrate, control reaction solution pH value to be 5, the CO produced 2 The gas enters the Ca(OH) 2 The absorption tank of the solution is absorbed;
[0039] (3) After filtering, concentrating, and cooling to room temperature, the copper citrate generated is put into a centrifuge for dehydration to obtain copper citrate crystals, and the separated waste liquid is discharged into the waste liquid pool for recycling;
[0040] (4) Put the obtained copper citrate crystals into a desiccator and dry them for 1.5 hours at 110° C. to remove part of the crystal wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com