A compound cell preparation, preparation method and use thereof
A cell preparation, cell technology, applied in cell culture active agents, biochemical equipment and methods, animal cells, etc., to reduce limb pain and coldness, and prolong amputation time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
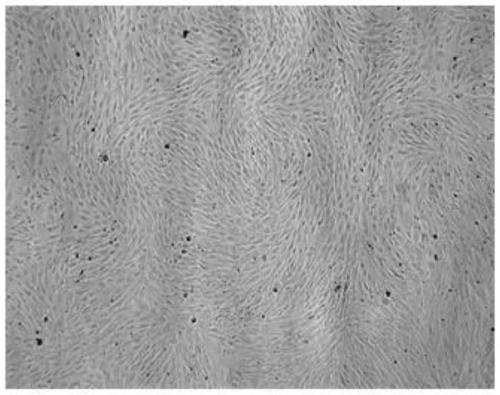
[0062] Example 1: Preparation of autologous ADSCs
[0063] 1.1 Fat collection
[0064] Before the collection, the donor should have a physical examination, no tumor history, no virus infection, no mycoplasma infection, and the fat collection should be collected in a professional medical collection institution; the specific method is abdominal subcutaneous liposuction 50mL. Immediately after collection, place them in a 125mL ice-bath sterile bottle (model 2019-0250, manufacturer Nalgene), which contains 50mL of preservation solution.
[0065] The preservation solution is DMEM / F12 with the following components added:
[0066] 50ug / mL gentamicin sulfate;
[0067] 10% sodium heparin anticoagulant.
[0068] 1.2 Isolation of fat cells
[0069] Remove small blood vessels and connective tissue, cut them into fluid state, and rarely see particles; resuspend in 2 times the volume of medical saline (containing 50ug / mL gentamicin sulfate), centrifuge at 500g for 10 minutes, and take t...
Embodiment 2
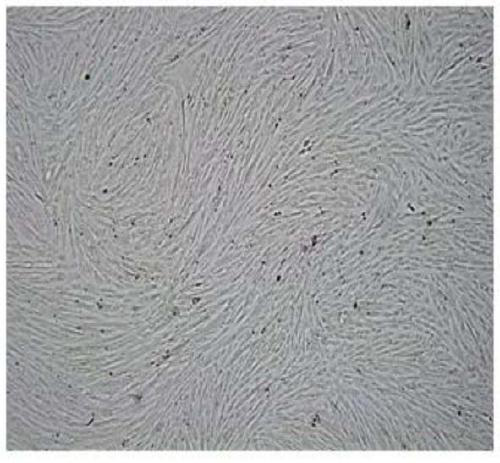
[0076] Example 2: Preparation of EPCs derived from ADSCs
[0077] Resuspend P2 generation ADSCs in ADSCs medium, adjust the cell density to 10000 / cm2, inoculate into 175 cm2 tissue culture flask, culture for 24 hours, change EPCs medium, continue to culture for 6 days, and harvest P0 generation EPCs.
[0078] The EPCs culture medium is DMEM / F12 containing the following components:
[0079] 5% animal-derived component-free serum replacement;
[0080] 10-8mol / L dexamethasone;
[0081] 20ng / mL recombinant human vascular endothelial growth factor;
[0082] 5ng / mL recombinant human basic fibroblast growth factor;
[0083] 5ng / mL insulin-like growth factor-1.
Embodiment 3
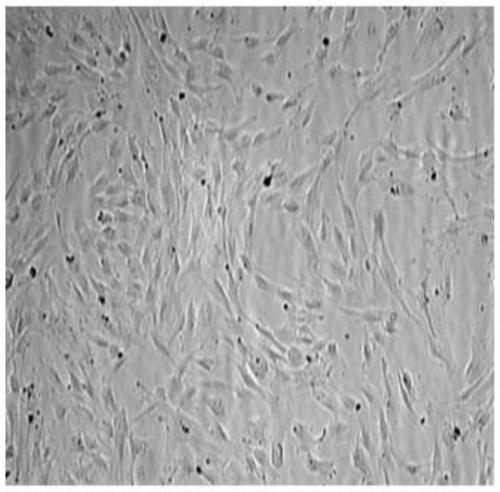
[0084] Embodiment three: PRP preparation
[0085]Clinically collect 100mL of the recipient’s own sodium citrate anticoagulated peripheral blood, centrifuge at 150g for 10 minutes, collect the supernatant and some red blood cells; take another 240g and centrifuge for 5 minutes, discard the upper 30% of the supernatant, and collect the middle layer and the upper part of the red blood cell layer For the buffy coat, about 40mL of PRP is reserved.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com