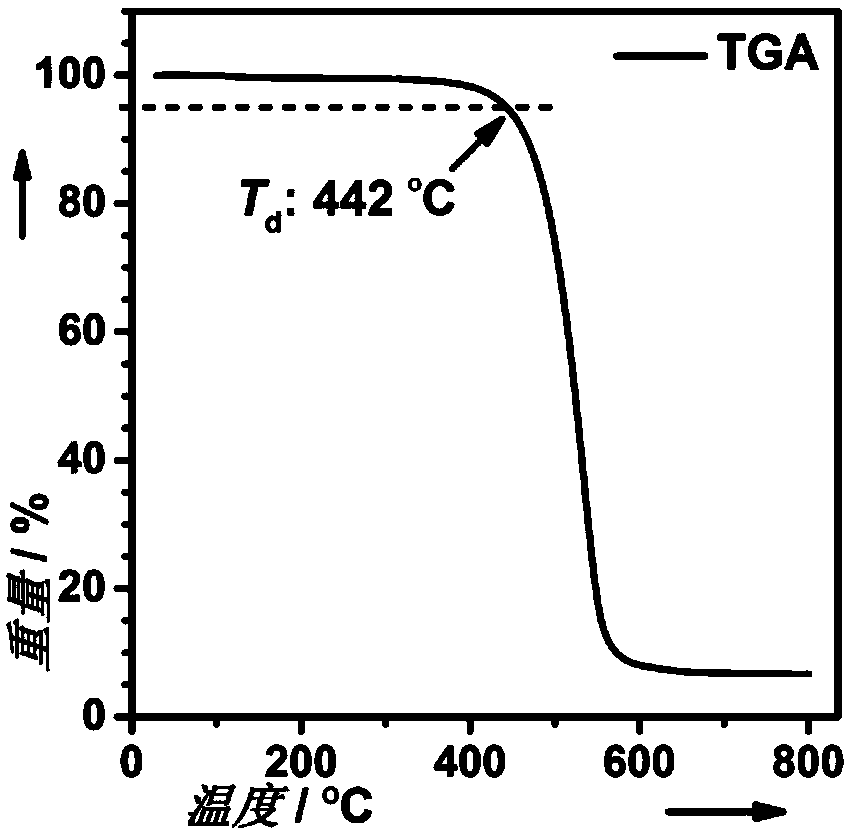
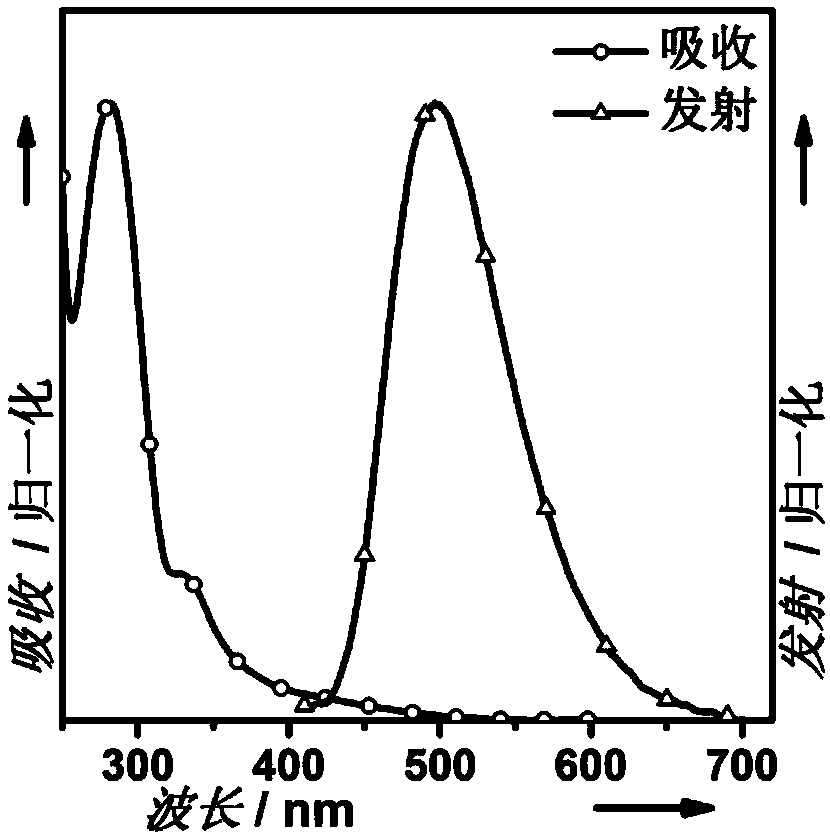
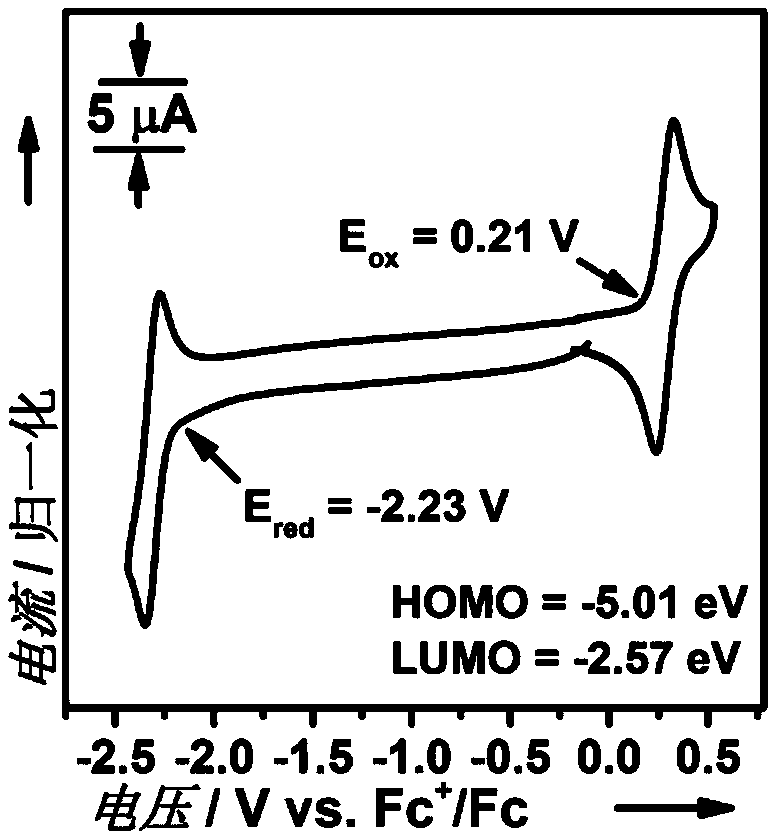
Interruption conjugation-based donor/acceptor type intramolecular exciplex light-emitting material and application thereof in preparation of organic light-emitting diode device
A technology of luminescent material and donor-acceptor type, applied in luminescent materials, electro-solid devices, semiconductor devices, etc., can solve the problems of low device efficiency and inability to use triplet state, achieve single structure determination, facilitate injection and transmission, The effect of good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] This embodiment P 1 The preparation comprises the following preparation steps:
[0046] m 1 Synthesis of: M 1 Prepared by Suzuki coupling. In a 100mL round bottom flask, 4,4'-dibromodiphenyl ether (5mmol, 1.63g), 4-cyanophenylboronic acid (5mmol, 735mg), and tetrakistriphenylphosphine palladium (0.1mmol, 115mg) were dissolved in 40mL of toluene and 20mL of potassium carbonate aqueous solution (2.0mol L -1 ), stirred and refluxed at 90°C for 24 hours under nitrogen protection. After the reaction, extract with dichloromethane, concentrate the extract by rotary evaporation, and separate by column chromatography (petroleum ether:dichloromethane=4:1, volume ratio) to obtain a white solid (872 mg, yield: 50%). Mass Spectrum MALDI-TOF(m / z)[M + ]: The test value is 349.16, and the theoretical value is 349.01.
[0047]
[0048] P 1 Synthesis of :P 1 Prepared by Ullmann coupling. In a 100mL round bottom flask, M 1 (5mmol, 1.75g), 9,10-dihydro-9,9-dimethylacridine (5...
Embodiment 2
[0051] This embodiment P 2 The preparation comprises the following preparation steps:
[0052] m 2 Synthesis of: M 2 Prepared by Ullmann coupling. In a 100mL round bottom flask, 4,4'-dibromodiphenyl ether (30mmol, 9.77g), 9,10-dihydro-9,9-dimethylacridine (5mmol, 1.05g), three (di Benzylideneacetonate)dipalladium (0.1mmol, 92mg), 1,1'-bis(diphenylphosphino)ferrocene (0.2mmol, 111mg), potassium tert-butoxide (10mmol, 1.12g) were dissolved in 40mL In toluene, stirred and refluxed at 110°C for 10 hours under the protection of nitrogen. After the reaction, extract with dichloromethane, concentrate the extract by rotary evaporation, and separate by column chromatography (petroleum ether:dichloromethane=50:1, volume ratio) to obtain a white solid (1.03g, yield: 45%). Mass Spectrum MALDI-TOF(m / z)[M + ]: The test value is 455.17, and the theoretical value is 455.09.
[0053]
[0054] m 3 Synthesis of: M 3 Prepared by Suzuki coupling. In a 100mL round bottom flask, M 2 (5...
Embodiment 3
[0059] This embodiment P 3 The preparation comprises the following preparation steps:
[0060] m 4 Synthesis of: M 4 Obtained by nucleophilic substitution reactions. In a 250mL round bottom flask, dissolve 4,4'-dibromodiphenyl ether (30mmol, 9.77g) in freshly distilled tetrahydrofuran, and add n-butyllithium (33mmol, 2.4M, 13.75mL), stirring was continued at -78°C for 4 hours. Bis(mesityl)boron fluoride (30mmol, 8.05g) was dissolved in freshly distilled tetrahydrofuran, and added dropwise to the reaction system, and stirred at room temperature for two days under nitrogen protection. Pour into water to quench the reaction, extract with dichloromethane, concentrate the extract by rotary evaporation, and separate by column chromatography (petroleum ether:dichloromethane=20:1, volume ratio) to obtain a white solid (6.71g, yield: 45% ). Mass Spectrum MALDI-TOF(m / z)[M + ]: The test value is 496.37, and the theoretical value is 496.16.
[0061]
[0062] P 3 Synthesis of :...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com