Preparation method of fluorescence phase-change material
A phase change material, phase change core material technology, applied in luminescent materials, nanotechnology for materials and surface science, heat exchange materials, etc. Actual needs and other issues to achieve the effect of preventing leakage and corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0025] (1) Preparation of MOFs / CQDs materials:
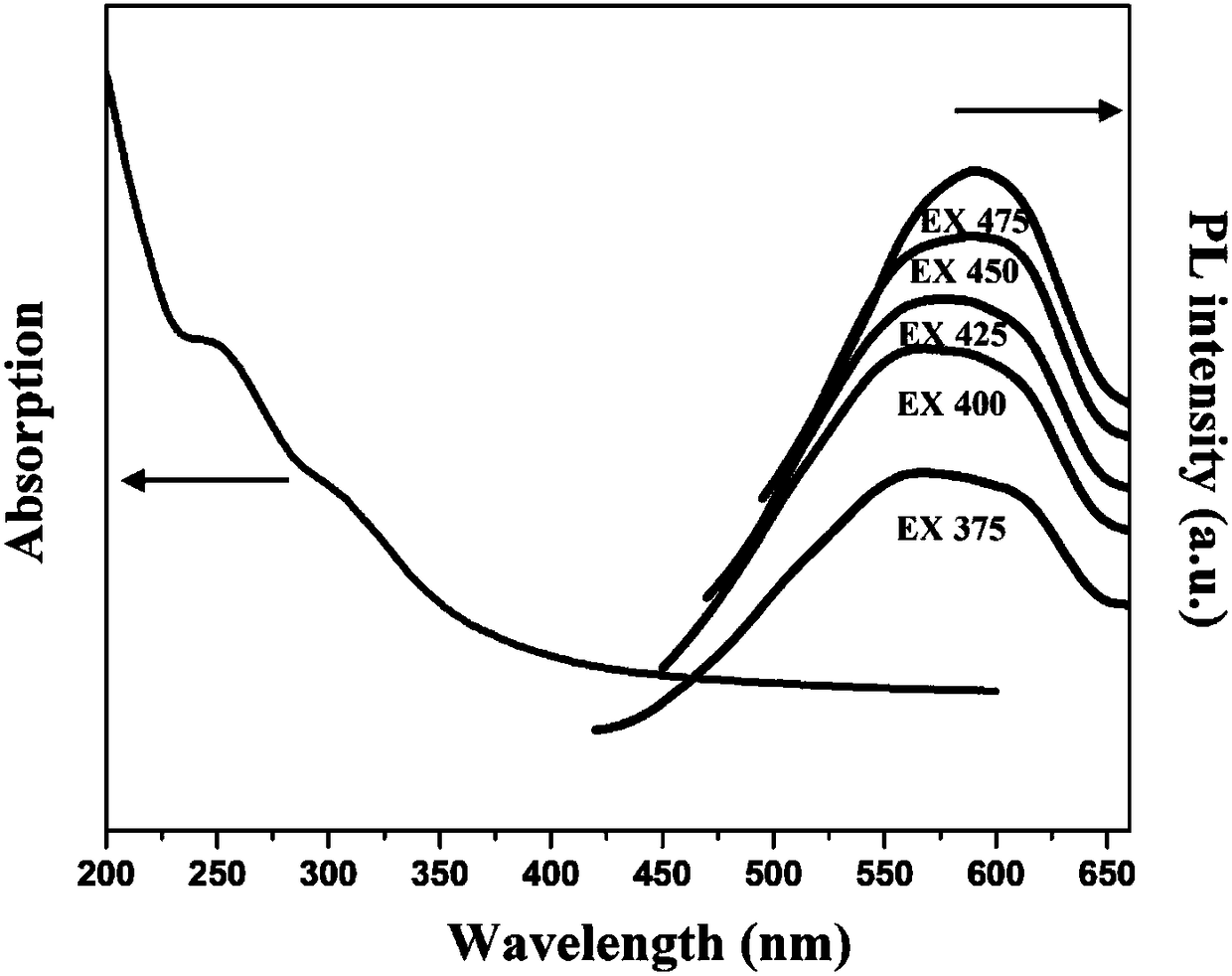
[0026] Mix 5g of sodium hydroxide and 100mL of acetone, add 3mL of divinylbenzene, stir vigorously at room temperature for 4h, then stand at room temperature, normal pressure, and air for 72h, and wash the product with 1mol / L hydrochloric acid solution Multiple times, unreacted sodium hydroxide was removed, washed with deionized water until neutral, and then dried in a freeze-drying oven at -75°C for 24 hours to obtain CQDs powder. where TEM as figure 1 As shown, UV-vis and PL as figure 2 shown.
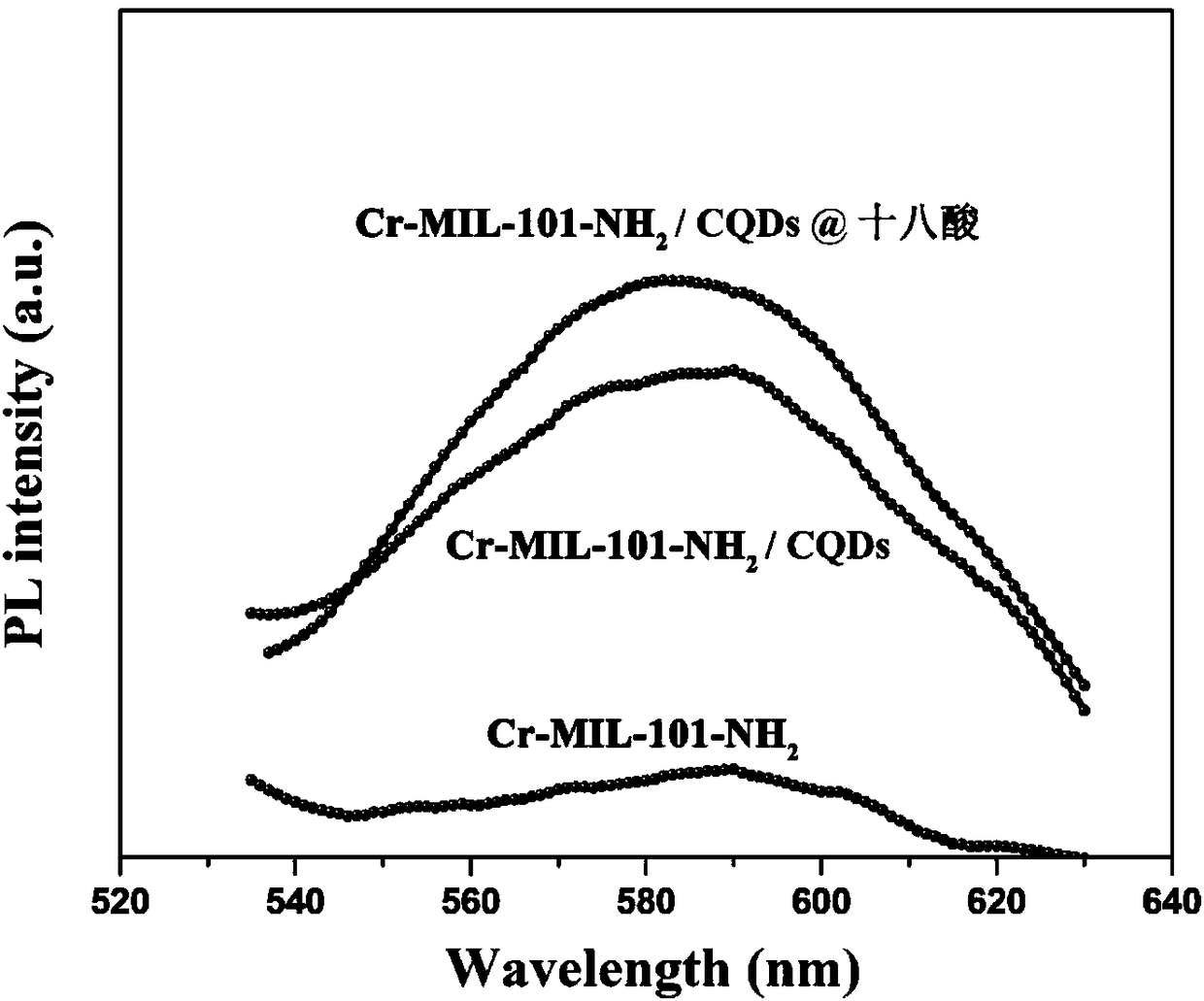
[0027] Disperse 8mmol of chromium nitrate nonahydrate, 8mmol of 2-aminoterephthalic acid, and 20mmol of sodium hydroxide in 60mL of deionized water, then add 200mg of CQDs powder, stir at room temperature for 2 hours, then move it to a reaction kettle, and set at 150 Incubate at ℃ for 12 hours, filter and wash, and dry in a vacuum oven at 80℃ for 24 hours to obtain Cr-MIL-101-NH 2 / CQDs carrier material.
[0028] (2) Preparation...
Embodiment example 2
[0031] (1) Preparation of MOFs / CQDs materials:
[0032] Mix 20g of potassium hydroxide and 150mL of acetaldehyde, add 6mL of styrene, stir vigorously at room temperature for 5h, then stand at room temperature, normal pressure, and air for 80h, and wash the product with 2.5mol / L hydrochloric acid solution Multiple times, unreacted potassium hydroxide was removed, washed with deionized water until neutral, and then dried in a freeze-drying oven at -75°C for 24 hours to obtain CQDs powder.
[0033]Disperse 2.1 mmol of aluminum nitrate and 3.2 mmol of 2-aminoterephthalic acid in 50 mL of N,N-dimethylformamide, then add 100 mg of CQDs powder, stir at room temperature for 2 hours, and then transfer it to an oil bath , reacted at 120°C for 72 hours, filtered and washed, and dried in a vacuum oven at 80°C for 24h to obtain Al-MIL-53-NH 2 / CQDs carrier material.
[0034] (2) Preparation of fluorescent phase change materials:
[0035] Dissolve 0.30 g of paraffin in 30 mL of absolute...
Embodiment example 3
[0037] (1) Preparation of MOFs / CQDs materials:
[0038] Mix 20g of sodium carbonate and 120mL of benzaldehyde, add 6mL of acrylic acid, stir vigorously at room temperature for 4h, then stand at room temperature, normal pressure, and air for 96h, and wash the product with 3mol / L hydrochloric acid solution several times, Unreacted sodium carbonate was removed, washed with deionized water until neutral, and then dried in a freeze-drying oven at -75°C for 24 hours to obtain CQDs powder.
[0039] Fully dissolve 1.7mmol zirconium chloride and 1.7mmol 2-aminoterephthalic acid in 25mL N,N-dimethylformamide and deionized water mixed solution (N,N-dimethylformamide and deionized water The volume ratio is 199:1), add 850mmol acetic acid, then add 100mg CQDs powder, ultrasonically disperse at 60°C for 0.5 hours, then react at 120°C for 24 hours, filter and wash, and put in a vacuum oven at 80°C After drying for 24h, Zr-UIO-66-NH was obtained 2 / CQDs carrier material.
[0040] (2) Prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
| Latent heat of phase change | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com