Novel polymer-bonded vascular blocker as well as preparation method and medical application thereof
A polymer and bonded drug technology, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to achieve the effects of improving cycle stability, improving distribution, and improving tumor inhibition effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The present invention also provides a preparation method of amino acid block copolymer, comprising:
[0054] There is the monoaminopolyethylene glycol of formula (III) or formula (IV) structure and gamma-benzyl-L-aspartic acid ester-N-internal carboxylic anhydride monomer stirring reaction in organic solvent, obtains with A compound with a protecting group; reacting the compound with a protecting group with ethanolamine to obtain a block copolymer of formula (VII); or having a bisaminopolyethylene glycol of formula (V) or formula (VI) in combination with γ -Benzyl-L-aspartic acid-N-internal carboxylic acid anhydride monomer is stirred and reacted in an organic solvent to obtain a compound with a protecting group; the compound with a protecting group is reacted with ethanolamine to obtain a compound having the formula (VIII) block copolymers of structure;
[0055]
[0056] where R 1 Independently selected from C1~C40 alkyl groups or composed of sulfhydryl groups, su...
Embodiment 1
[0069] Add 5.00 g of polyethylene glycol monomethyl ether having a structure of formula (III) with a number average molecular weight of 5000 to the dry reaction bottle, and remove water with 80 mL of anhydrous toluene at 130° C. for 3 hours, then vacuum dry The remaining toluene; the obtained solid was dissolved in 50 mL of dry N,N-dimethylformamide to obtain the first solution; 3.50 g of γ-benzyl-L-aspartate-N-endocarboxylic anhydride Dissolve in 40mL of dry N,N-dimethylformamide to obtain the second solution; in a nitrogen atmosphere, mix the first solution and the second solution, stir and react at room temperature under nitrogen protection conditions for 48h; then increase the temperature At 35°C, 10 mL of acetic anhydride was added to continue the reaction for 24 h. After the reaction, most of the N,N-dimethylformamide and unreacted acetic anhydride were removed under reduced pressure, then settled with ether, filtered by suction, and dried to obtain a block copolymer wit...
Embodiment 2
[0075] Add 0.69 g of the block copolymer having the structure of formula (VII-a) prepared in Example 1, DMXAA (0.37 g) and DMAP (120 mg) into the dry reaction flask, and vacuumize for 12 h. Then 10 mL of dry N,N-dimethylformamide was added to dissolve; DIC (1.3 g) was added with a syringe, and the reaction was stirred at room temperature under nitrogen protection for 24 h. After the reaction, settle with excess diethyl ether, wash, suction filter, and dry, the obtained crude polymer bonded drug is dissolved in N,N-dimethylformamide, and then dialyzed in pure water for 72 hours. Water 10 times, the dialysate was purified by high-speed centrifugation, and then passed through a 220nm filter membrane. Finally, the polymer-bonded drug with the structure of formula (I) is obtained by freeze-drying.
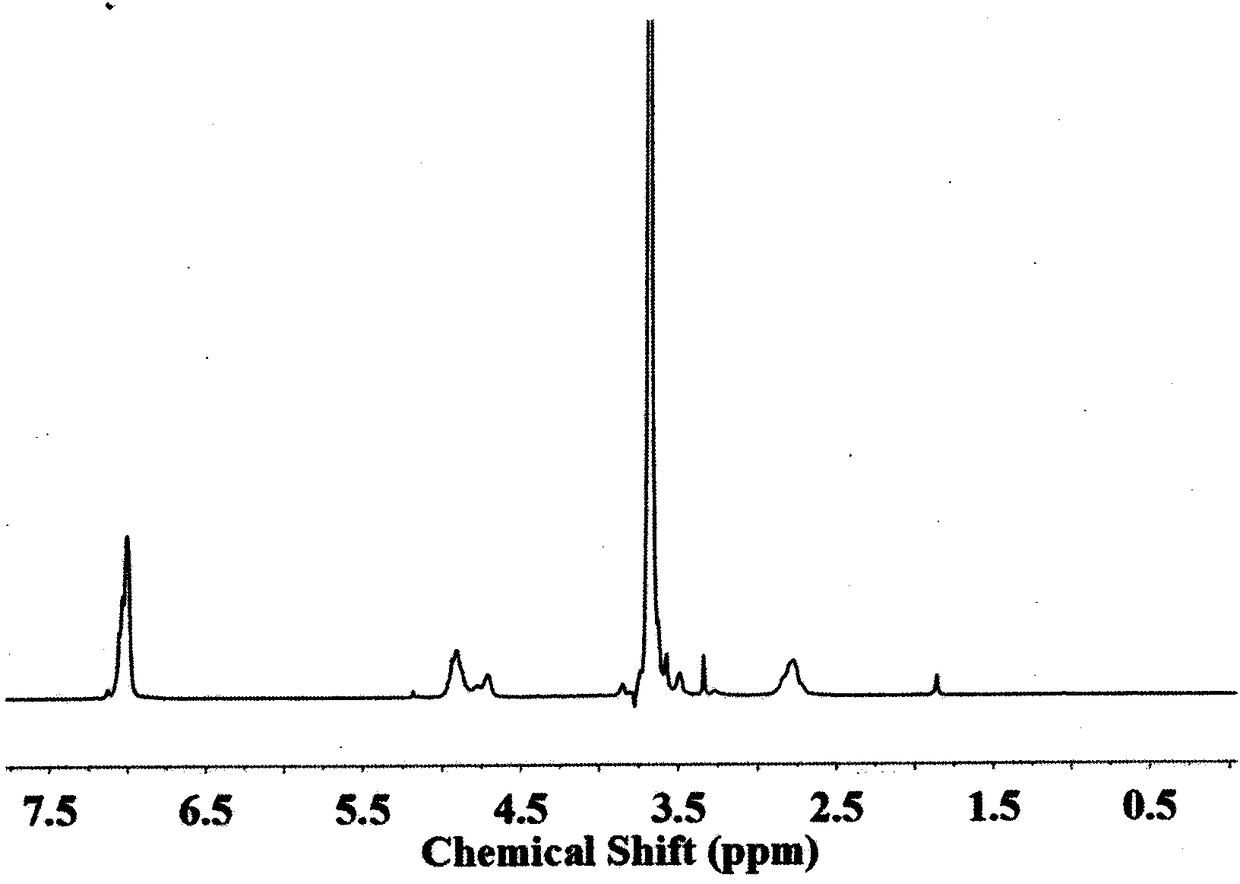
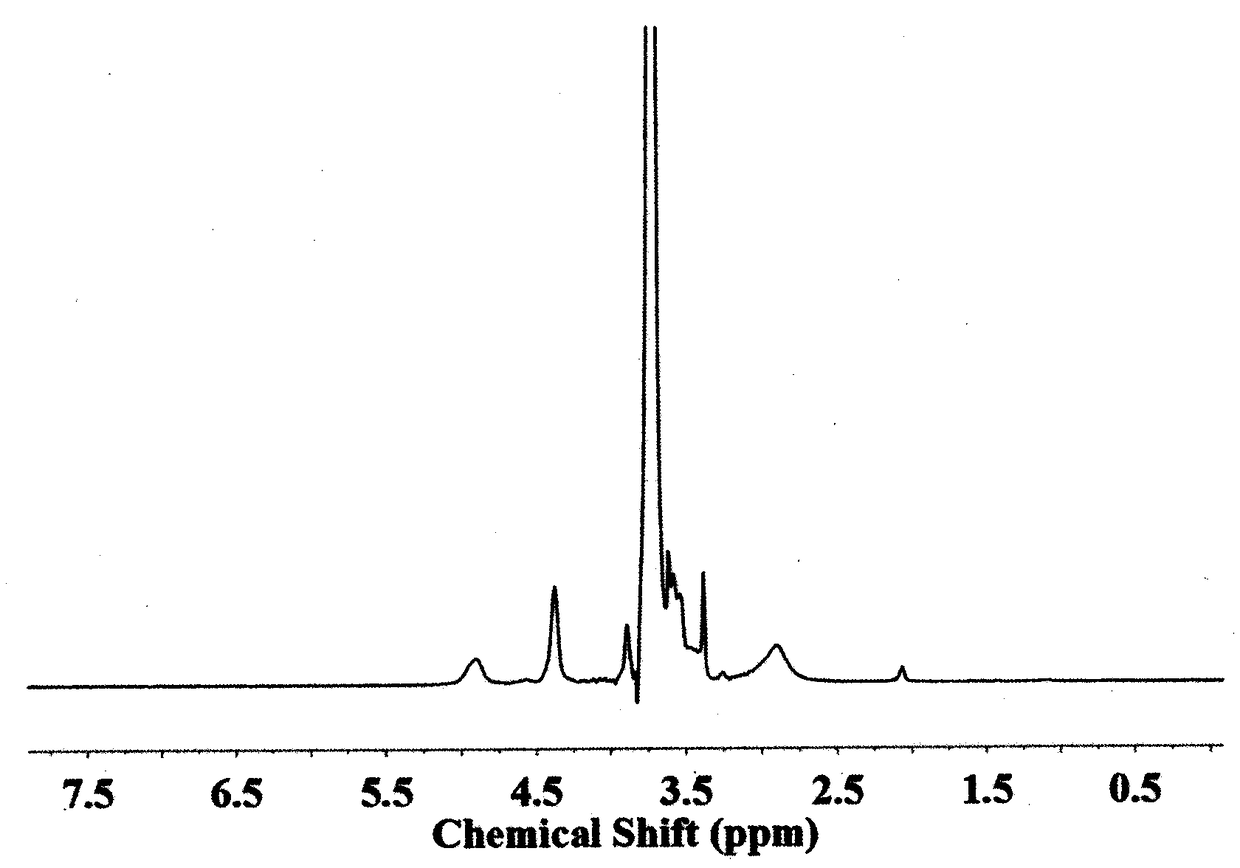
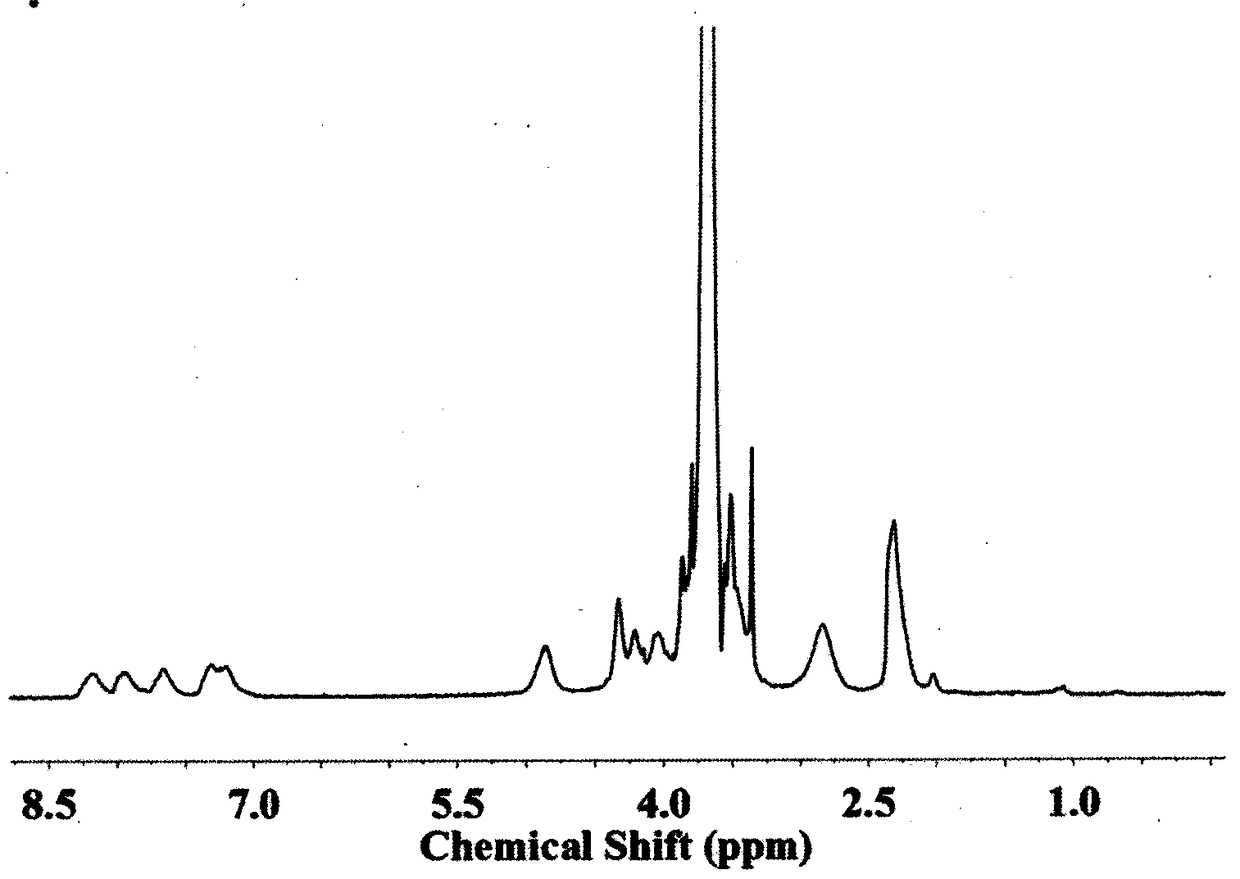
[0076] Carry out nuclear magnetic resonance analysis to the block copolymer that obtains, the result sees image 3 , image 3 The H NMR spectrum of the polymer-bonded drug prepared i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com