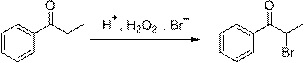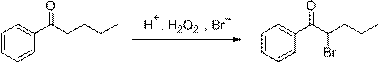Preparation method of alpha-bromo aromatic ketone compounds
A technology for aromatic ketones and compounds, which is applied in the field of preparation of α-bromoaromatic ketones, can solve the problems of high price and environmental pollution, and achieve the effects of high selectivity, reduced production costs, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] At room temperature, add 16.2g (0.1mol) of valerophenone and 30.9g (0.3mol) of sodium bromide into a 500 mL round-bottomed flask, stir well, then add 24g (0.2mol) of 30% hydrochloric acid, and slowly Add 19g (0.15mol) of 30% hydrogen peroxide dropwise. After the dropwise addition, continue to react for 1~2h. After the reaction is monitored by TLC, stop stirring, let stand to separate layers, wash with saturated sodium carbonate and saturated saline respectively, and combine organic phase, concentrated and dried over anhydrous magnesium sulfate to obtain α-bromovalerophenone, a bright yellow oily liquid with a yield of 95% and a purity of 98% (HPLC method).
[0024]
Embodiment 2
[0026] Add 19.6g (0.1mol) of p-chlorovalerone to a 500mL round bottom flask equipped with a reflux condenser, add 50mL of methanol and stir to dissolve, then add 30.05g (0.35mol) of sodium bromide, stir well, Add 25.3g (0.25mol) of 36% hydrochloric acid, and slowly add 25.3g (0.2mol) of 30% hydrogen peroxide dropwise. After the dropwise addition, raise the temperature to 70~75°C and continue the reaction for 1~2h. TLC monitors the reaction after the end , stop stirring, let stand to separate layers, wash with saturated sodium carbonate and saturated brine respectively, combine the organic phases, concentrate and dry over anhydrous magnesium sulfate to obtain p-chloro α-bromovalerophenone, yellow oily liquid, yield 96 %, purity 96% (HPLC method).
Embodiment 3
[0028] At room temperature, add 13.4g (0.1mol) of propiophenone and 41.2g (0.4mol) of sodium bromide to a 500 mL round bottom flask respectively, after stirring evenly, add 32.7g (0.1mol) of 30% sulfuric acid, and slowly Add 35.2g (0.25mol) of 27% hydrogen peroxide dropwise. After the dropwise addition, continue to react for 1~2h. After the reaction is monitored by TLC, stop stirring, let stand to separate layers, wash with saturated sodium carbonate and saturated saline respectively, and combine The organic phase was concentrated and dried over anhydrous magnesium sulfate to obtain α-bromopropiophenone, a yellow oily liquid with a yield of 94% and a purity of 96% (HPLC method).
[0029]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com