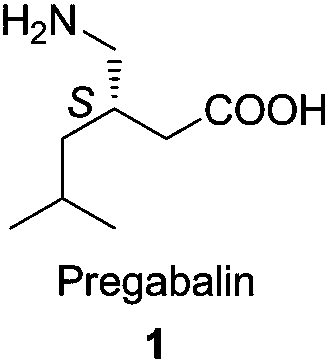
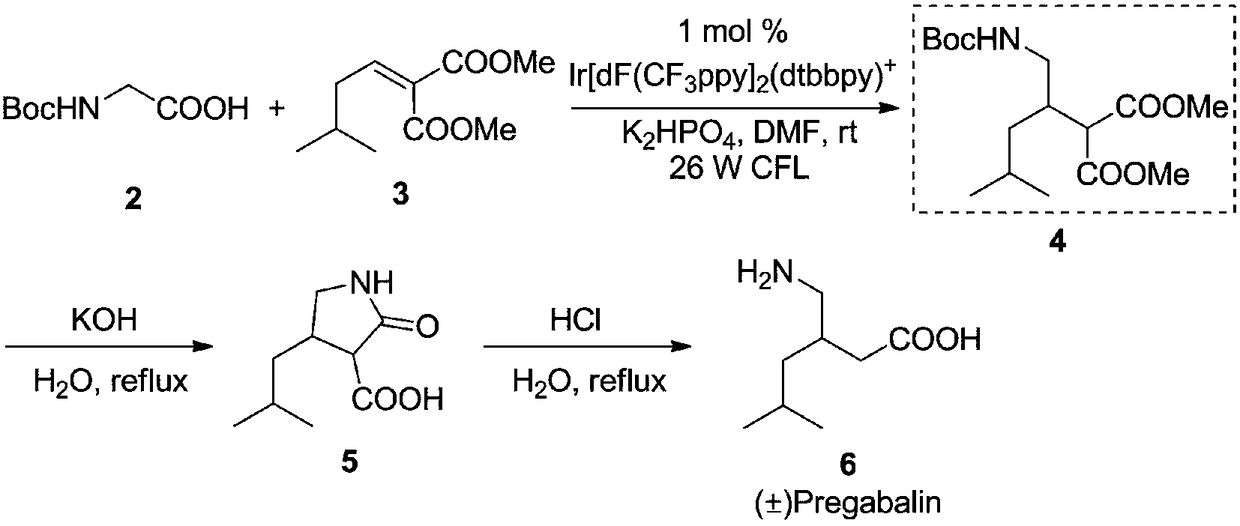
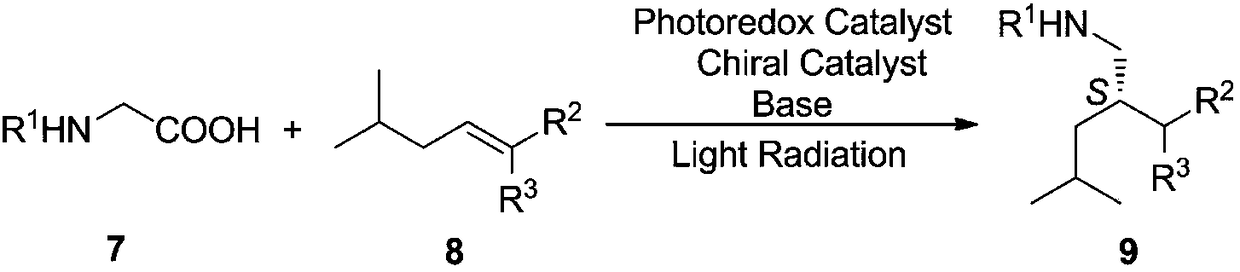
Preparation method of pregabalin
A technology of pregabalin and compounds, which is applied in the field of preparation of pregabalin, can solve problems such as hindering industrial application, prolonging the reaction route, increasing synthesis cost, etc., and achieve the effects of reducing synthesis cost, easily obtaining raw materials, and saving energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Photocatalytic asymmetric reaction
[0040] Under the protection of dry nitrogen, add formula 7 compound (R 1 = COOEt, 1.47g, 10.0mmol), formula 8 compound (R 2 =COOEt,R 3 =H, 1.56g, 10.0mmol), photoredox agent compound disodium salt of formula 41 (0.51g, 0.5mmol, 5 mol%), chiral organic catalyst compound of formula 60 (0.66g, 1mmol, 10mol%), three Ethylamine (1.21g, 12.0mmol) and acetonitrile 15mL were stirred for 5 minutes to degas and mix the reaction system. Then irradiate the quartz reaction bottle with a 26w green LED lamp, keep the distance from the quartz bottle at 0.5cm, control the reaction temperature at 20°C, stir the reaction for 36 hours, stop the irradiation, add 100mL water to the reaction system for dilution, and then extract with dichloromethane for 3 time, each 50mL, combined organic phases, dried over anhydrous sodium sulfate, suction filtered, evaporated to dryness, and the residue obtained formula 9 compound (R 1 =COOEt,R 2 =COOEt R 3 =H)...
Embodiment 2
[0045] (1) Photocatalytic asymmetric reaction
[0046] Under the protection of dry argon, add formula 7 compound (R 1 = Cbz, 2.09g, 10.0mmol), formula 8 compound (R 2 =COOMe,R 3 =H, 1.49g, 10.5 mmol), photoredox agent formula 10 compound (0.26g, 2.0mmol, 20mol%), chiral organic catalyst formula 50 compound (0.56g, 0.8mmol, 8mol%), potassium tert-butoxide (1.23g, 11.0mmol) and tetrahydrofuran 20mL, stirred for 5 minutes to degas and mix the reaction system. Then irradiate the quartz reaction flask with a 40w low-pressure mercury lamp (λ>200nm), keep a distance of 0.2cm from the quartz flask, control the reaction temperature at 10°C, stir the reaction for 24 hours, stop the irradiation, add 120mL of water to the reaction system for dilution, and then dilute with diethyl ether Extract 3 times, 60 mL each time, combine the organic phases, dry over anhydrous magnesium sulfate, filter with suction, evaporate the solvent to dryness, and the residue is subjected to flash silica gel...
Embodiment 3
[0050] (1) Photocatalytic asymmetric reaction
[0051] Under the protection of dry argon, add formula 7 compound (R 1 = PhNHCO, 1.95g, 10.0mmol), formula 8 compound (R 2 =COOPh,R 3 =H, 2.24g, 11.0mmol), photoredox agent compound tetrafluoroborate of formula 28 (0.10g, 0.25mmol, 2.5mol%), chiral organic catalyst compound of formula 58 (1.34g, 2mmol, 20mol%) , sodium bicarbonate (2.77g, 33.0mmol) and ethanol 30mL, stirred for 5 minutes to degas and mix the reaction system. Then irradiate the quartz reaction flask with a 100w blue LED lamp (λ=450nm), keep a distance of 3cm from the quartz flask, control the reaction temperature at 25°C, stir the reaction for 48 hours, stop the irradiation, add 150mL water to the reaction system for dilution, and then dichloro Methane was extracted 3 times, 75 mL each time, the organic phases were combined, dried with anhydrous calcium chloride, filtered with suction, and the solvent was evaporated to dryness. The residue was subjected to flash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com