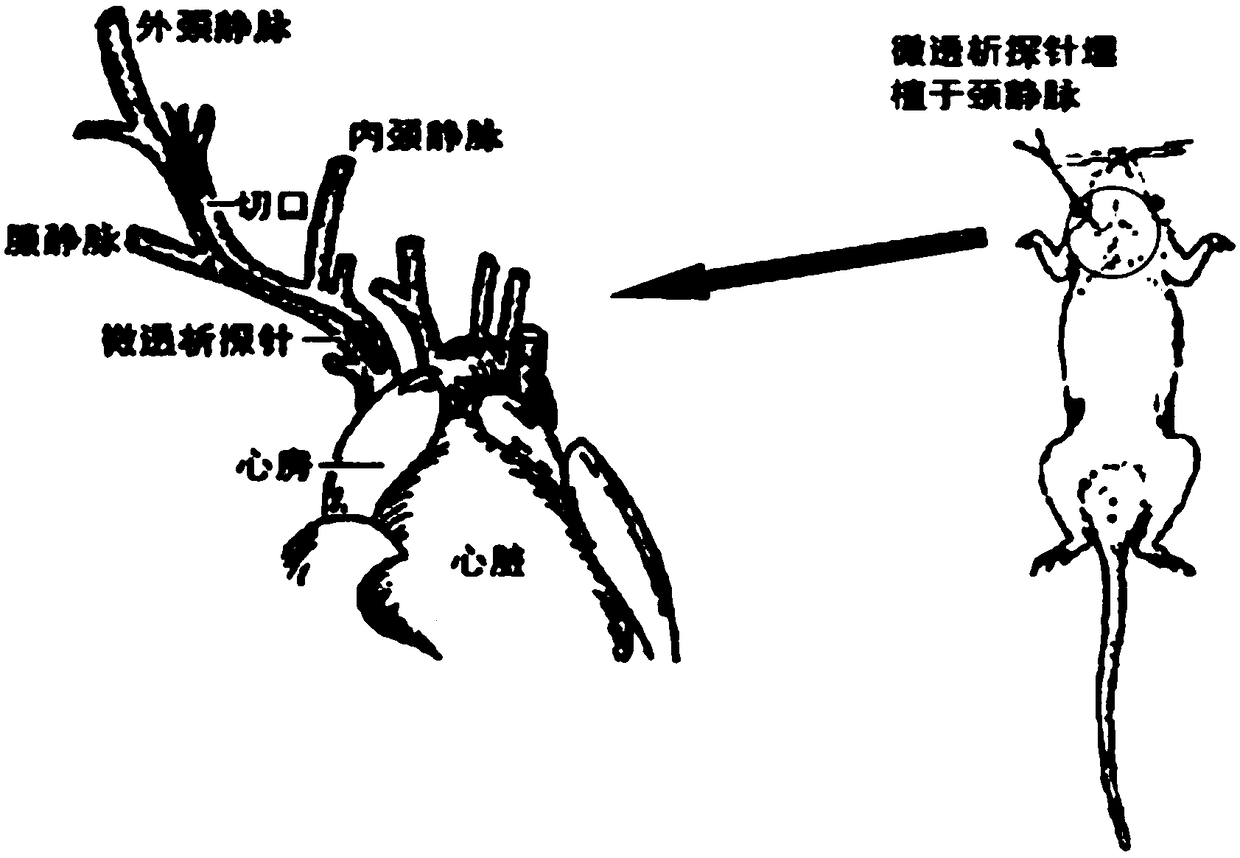
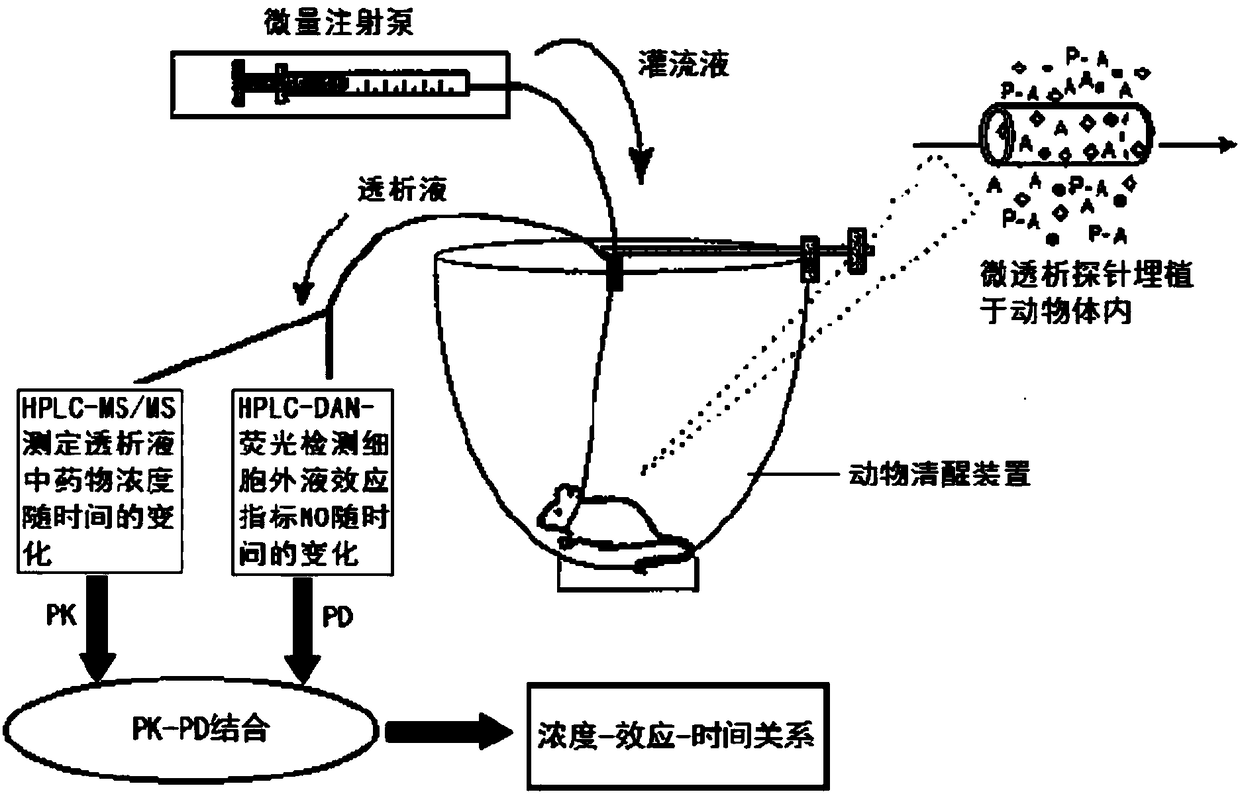
Blood dialysate detection method, and application thereof in pharmacokinetics-pharmacodynamics of pulse activating injection
A technology of Shengmai injection and hemodialysis, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1. Reagents and instruments
[0052] 1. Drugs and reagents
[0053] The reference substances ginsenoside Rg1, ginsenoside Rg2, ginsenoside Re, ginsenoside Rf, ginsenoside Rb1, ginsenoside Rd and ginsenoside Rc were purchased from Shanghai Ronghe Pharmaceutical Technology Development Co., Ltd. Shengmai injection (concentrations of ginsenosides Rg1, Rg2, Re, Rf, Rb1, Rd and Rc were determined to be 140.8, 22.5, 79.8, 38.3, 140.0, 25.4 and 79.4 μg / mL, respectively) was produced by Changshu Leiyunshang Pharmaceutical Co., Ltd. Limited offer. Isoproterenol (Isoproterenol, ISO) was purchased from Sigma. The internal standard digoxin (digoxin) was purchased from China National Institutes for Food and Drug Control. Normal saline for injection was purchased from the Affiliated Hospital of Zhejiang University. Methanol and acetonitrile were chromatographically pure (Merck), acetic acid was chromatographically pure (Tedia), and deionized water was prepared by a Milli-Q ultrapu...
Embodiment 2
[0082] Embodiment 2 pharmacokinetic research results and discussion
[0083] 1. Methodological validation
[0084] 1. Optimization of mass spectrometry conditions
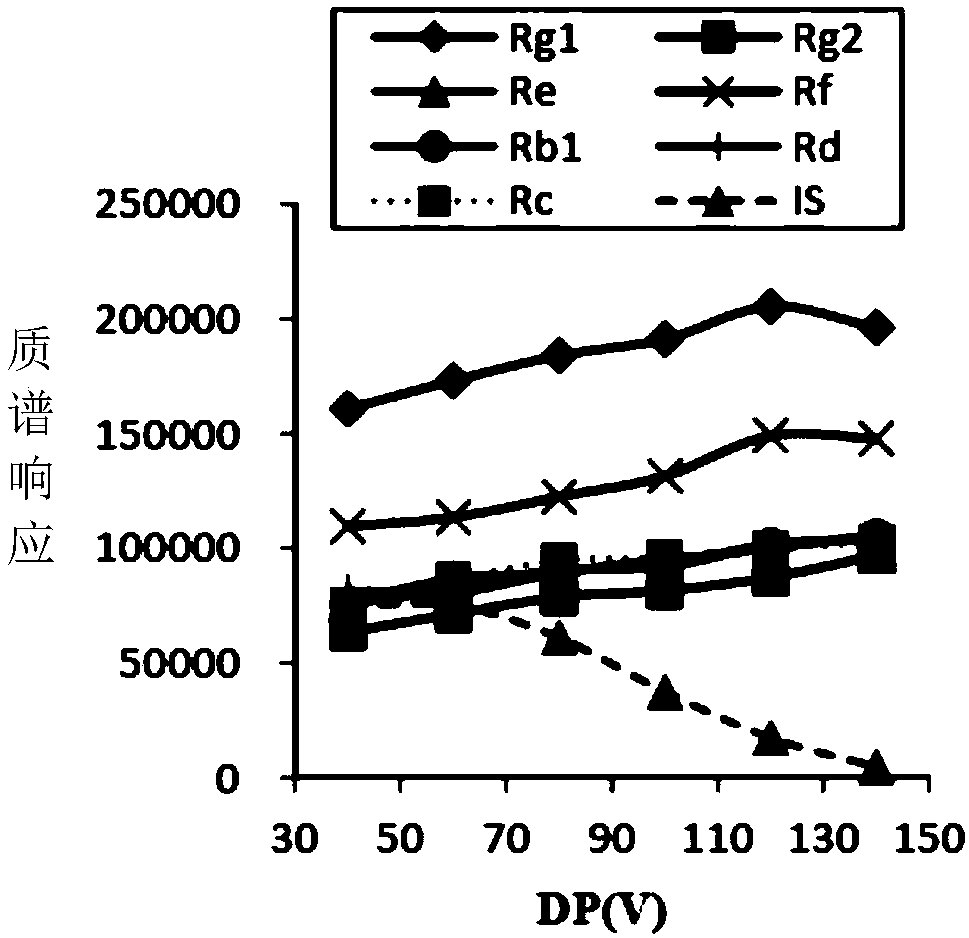
[0085] Optimization of mass spectrometry parameters: Prepare mixed standard solutions of ginsenosides Rg1, Rg2, Re, Rf, Rb1, Rd, Rc and internal standard (IS) with physiological saline, and perform mass spectrometry parameters declustering voltage (DP), entrance voltage (EP) respectively. ), collision energy (CE), and exit voltage (CXP), the optimal parameters are mainly selected according to the mass spectrum response size (intensity), and the optimization results are as follows: image 3 , Figure 4 , Figure 5 and Figure 6 shown.
[0086] According to the optimization results in the figure above, the DPs of ginsenosides Rg1, Rg2, Re, Rf, Rb1, Rd, Rc and internal standard (IS) were finally determined to be 120, 140, 140, 120, 140, 140, 140 and 60V, respectively, and EP 15, 15, 9, 15, 9, 9, 13, and 7V respe...
Embodiment 3
[0136] Embodiment 3 Pharmacodynamic research results and discussion
[0137] 1. Methodological validation
[0138] 1. Linear
[0139] Accurately absorb a certain amount of NO 3 - Standard stock solution, diluted with normal saline to a series of NO concentrations of 0, 2.5, 5.0, 10.0, 15.0, 20.0, 25.0, 30.0 and 40.0 μM 3 - The standard solution was subjected to HPLC-FLU analysis after being processed according to the method under item 2 of the fourth part of the above-mentioned embodiment 1. Will NO 3 - The peak area (y) of each standard solution concentration (x) and each reaction product NAT is carried out linear regression, obtains standard curve and is: y=240377.82x+123099.47, R 2 =0.9981, NO 3 - The physiological saline solution has a good linear relationship in the concentration range of 0-40.0μM.
[0140] 2. Precision and sample recovery
[0141] Accurately weigh 10 μL of the dialysate sample and treat it according to the method under item 2 of Part IV of Exa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com